Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (17): 2774-2781.doi: 10.12307/2024.366
Previous Articles Next Articles
Research and advance of hydrogel-promoted endometrial repair in intrauterine adhesions
Wu Haoming1, Wang Yao1, Chen Yuanmeng1, Zhu Huili2, Li Kainan1, Xiong Chengdong3, Hu Xulin1, 4
- 1Clinical Medical College/Affiliated Hospital of Chengdu University, Chengdu 610081, Sichuan Province, China; 2Department of Reproductive Medicine, Key Laboratory of Birth Defects and Related Diseases of Women and Children of Ministry of Education, West China Second University Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China; 3University of Chinese Academy of Sciences, Beijing 100049, China; 4State Key Laboratory of Biotherapy, Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2023-04-22Accepted:2023-06-05Online:2024-06-18Published:2023-12-16 -
Contact:Hu Xulin, PhD, Lecturer, Clinical Medical College/Affiliated Hospital of Chengdu University, Chengdu 610081, Sichuan Province, China; State Key Laboratory of Biotherapy, Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Wu Haoming, Clinical Medical College/Affiliated Hospital of Chengdu University, Chengdu 610081, Sichuan Province, China -
Supported by:Natural Science Foundation of Sichuan Province, No. 23NSFSC5880 (to HXL); Chengdu Medical Research Project, No. 2022004 (to HXL); Medical Science and Technology Project of Sichuan Provincial Health Commission, No. 21PJ127 (to WY); Chengdu Medical Research Project, No. 2022291 (to WY); National Natural Science Foundation of China, No. 81601258 (to ZHL); Chengdu Science and Technology Project, No. 2021-YF05-01612-SN (to ZHL)
CLC Number:
Cite this article
Wu Haoming, Wang Yao, Chen Yuanmeng, Zhu Huili, Li Kainan, Xiong Chengdong, Hu Xulin. Research and advance of hydrogel-promoted endometrial repair in intrauterine adhesions[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2774-2781.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
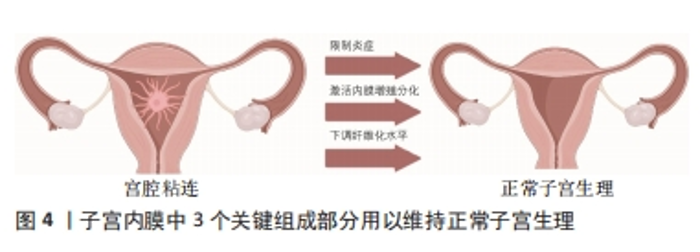
更为重要的是,水凝胶的上述性质可以在物理和化学两方面进行调节,因此在各种应用中获得极大的关注,例如组织工程、药物缓释和三维细胞培养。总而言之,水凝胶是用于包封和递送治疗物质的完美平台,已成为一种极有前途的子宫内膜修复策略。 2.2 水凝胶作用于宫腔粘连的指导依据及关键性能 宫内能否使用水凝胶主要决定于它的凝胶化时间、黏度、生物相容性、可降解性,其主要受到宫腔生理特性的影响。子宫会进行周期性排血,同时子宫内膜蠕动(子宫肌层会不停收缩,导致子宫内膜蠕动)引起腔内液体流动,为胚胎着床提供营养支持[31]。首先,水凝胶的凝胶化时间应该较短,在注射后可以短期进行凝胶化,更好地黏附在创面,但是需要注意平衡其机械性能和粘连性能[32-33]。其次,水凝胶前体黏度应该较低,这样便于运输到子宫内部[34]。一定良好的生物相容性可以保证药物/细胞更好地起作用。水凝胶应在体内缓慢降解,可以随着液体从子宫排除。理想状态的水凝胶可以提供药物、干细胞或生长因子的温和控制释放[35]。 2.2.1 宫腔粘连的生理基础和病理状态 子宫内膜大体可以分为基底层和功能层两层。内膜表面2/3为功能层,主要受到卵巢性激素影响进而发生周期变化,最终导致脱落。基底层为靠近子宫肌层的1/3内膜,不受卵巢性激素影响,不发生周期性变化。基底层主要包括基质、基底部分的腺体、支持血管系统及各种免疫细胞群,如自然的杀伤细胞(NK细胞)、中性粒细胞、巨噬细胞和淋巴细胞(T或B细胞)[19,36],基底层主要作用为在月经期间保持完整性并提供新生细胞来源,同时还可以更新月经后的功能层[37]。 宫腔粘连的主要表现为基底膜损伤、子宫内膜纤维化及瘢痕的形成。当基底层受到损伤后,子宫内膜的再生能力和相应的小血管活力下降,各种免疫细胞群体无法发挥其正常功能,同时纤维组织增殖,最终导致子宫内膜纤维化。其中,纤维组织取代正常内膜上皮,并伴有腺体的减少或消失,当纤维化程度足够高时,子宫腔/子宫颈管部分或完全消失[38],子宫无法发挥正常生理功能,导致难以受孕。 2.2.2 水凝胶用于宫腔粘连的指导依据 水凝胶可用于防治宫腔粘连复发的关键在于其本身的生物相容性、体内降解性、优秀的力学性能、不引起免疫反应、优异的促内膜修复特性和提高妊娠结果的能力[39]。 理想水凝胶应该具有可生物降解、安全、非炎症、非免疫原性、在关键的月经阶段持续存在、不需要缝合保持活性且快速和容易应用等特性[40-41],并且尽可能降低炎症发生、缓解子宫内膜纤维化过程。图4是维持正常子宫生理的3个关键,包括:限制炎症,防止组织破坏;激活子宫内膜干细胞的增殖分化;下调子宫内膜纤维化水平。"

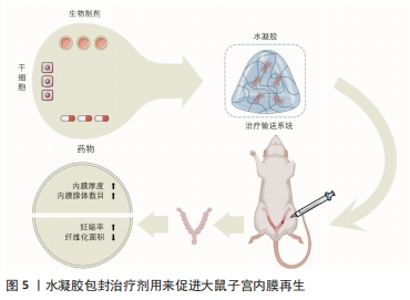
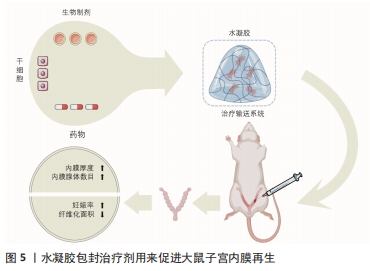
2.3 水凝胶类在宫腔粘连中的应用 天然聚合物水凝胶是来源于自然界的聚合物形成的水凝胶,例如来源于甲壳类动物的壳聚糖、来源于褐藻类的海藻酸钠及来源于动物皮的明胶,除此之外还有透明质酸、纤维素、胶原蛋白等。天然聚合物水凝胶在内膜形成、细胞增殖生长和形态维持方面具有良好的生物相容性,同时也可以吸水膨胀将宫腔内部支撑分离,但其力学性能较差、降解性无法控制[42]。合成水凝胶是以聚乙二醇、聚乙烯醇、聚乳酸、聚羟基乙酸、聚乳酸-羟基乙酸共聚物等为代表的聚合物,通过小分子单体或者合成高分子制备的一种材料,可以通过改变聚合度来改变水凝胶的特性,通常加入双官能团单体进行原位交联反应,也是目前比较热门的一类材料。与天然聚合物水凝胶相比,合成聚合物水凝胶具有较高的力学性能,可作为承重材料;然而,合成聚合物的细胞相容性较差,很容易引起无菌性炎症和肿胀并发症,这也是需要注意和提升的地方。 水凝胶类物质的低界面张力及黏附性能可以减少其对组织的刺激,同时还可以提供更为良好的扩散和更长的保留时间[28],因此,在近些年水凝胶类物质已成为治疗宫腔粘连或促进子宫内膜修复再生的最具影响力和吸引力的材料。高生物相容性的水凝胶不仅是防止宫内黏附的物理障碍,而且还可以作为输送系统提供多种治疗成分,包括药物、生物制剂、干细胞(图5),同时还可以通过化学/物理交联方法将水溶性或亲水聚合物交联起来提高其性能。根据原料的不同,大致可将水凝胶类物质分为透明质酸水凝胶、泊洛沙姆(Poloxamer)水凝胶和合成/复合水凝胶。"
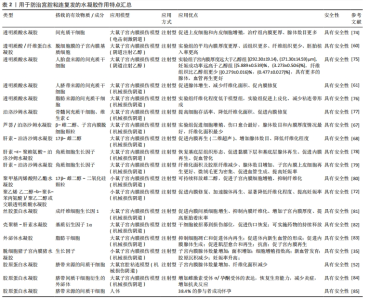
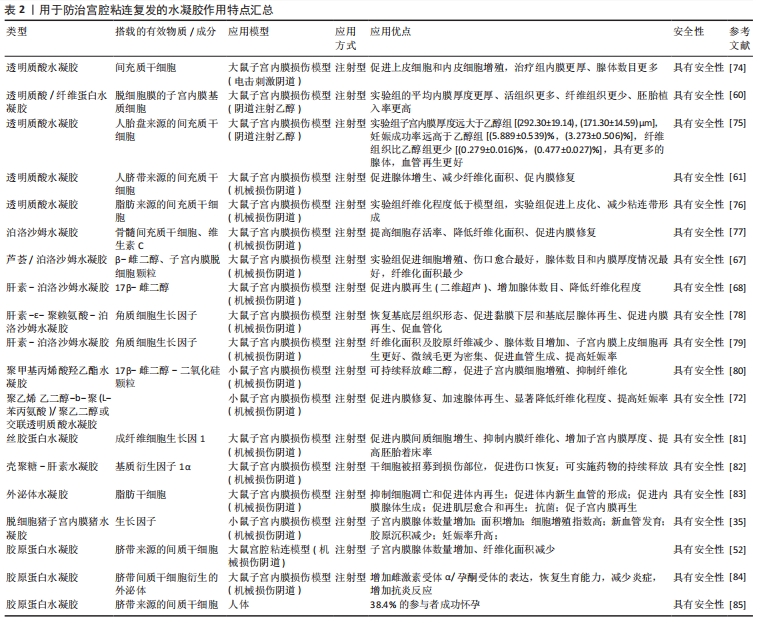
2.3.1 透明质酸水凝胶 透明质酸是生物医用水凝胶中天然聚合物的代表[43],在体内多存在于关节、皮肤和结缔组织[44],主要参与炎症调节、血管再生以及皮肤组织再生等,可以调节细胞的增殖分化、迁移情况[45-47]。优秀的生物相容性、可降解性、低免疫原性等促使透明质酸在生物医学中发挥着重要作用,被广泛用于骨科、眼科、鼻科、美容医学和癌症治疗中[48-49]。 透明质酸在体内为细胞提供良好的生长环境,同时透明质酸酶广泛存在于子宫内膜和腹腔,可以完全降解透明质酸[50]。透明质酸水凝胶在宫腔内部时可以通过下调炎症反应及促进血管再生来降低宫腔粘连的复发率[48],因此透明质酸水凝胶在宫腔粘连预防中扮演着越来越重要的角色。通过制备方式可将其分为两类——传统透明质酸水凝胶和交联透明质酸水凝胶。可简单将交联方法分为物理交联与化学交联两种方式。物理交联包括聚电解质络合和离子间的相互作用,如氢键范德华力等形成水凝胶,也可通过改变条件(如pH值、温度等)来形成水凝胶。物理交联形成水凝胶的交联条件通常比较温和,这也是目前交联形成水凝胶最常用的交联方式。化学交联指的是将具有官能团的交联剂添加到凝胶状态的聚合物稀溶液中,产生化学反应来促进水凝胶的形成,例如常见的希夫碱的偶联、酰肼-醛偶联等,通过共价键将网络中的聚合物链连接而成。与物理交联相比,化学交联形成的凝胶的机械性能更强,但是存在有毒交联剂难以去除的问题。 (1)传统透明质酸水凝胶:透明质酸是一种天然亲水性多糖,在机体组织中广泛存在,常见于羊水、胎盘等[51],同时它也是细胞外基质的主要成分。通过将透明质酸制备成水凝胶来促进内膜上皮细胞增殖、黏附,进而控制创伤面积,降低宫腔粘连的复发率,近些年不断有人将其引入宫腔粘连模型中并且取得了较好结果。 LIU等[52]利用透明质酸水凝胶搭载间充质干细胞作为宫腔粘连防治材料,体外细胞实验中搭载间充质干细胞水凝胶中的细胞增殖最快,且能促进人子宫内膜上皮细胞系AN3CA的生长;扫描电镜分析显示,细胞外空间可见胶原纤维,4 h后可观察到小的细胞团,24 h后观察到更大的细胞聚集团,并可以很好地整合到水凝胶中。将间充质干细胞/ 透明质酸水凝胶注射进入小鼠宫腔粘连模型中,组织切片结果表明,用水凝胶治疗组可明显减少纤维组织增生、增加子宫内膜厚度,同时显示出良好的组织结构和明显的新生血管,再生的子宫内膜功能恢复较好。最后,将所有组别进行受孕处理,发现间充质干细胞/透明质酸组胎儿数量显著高于对照组。 干细胞凋亡常伴随着凋亡小体的释放。凋亡小体与组织再生、免疫调节相关[53-54],凋亡小体已经被证实可以促进骨修复和皮肤愈合[55-56]。XIN等[57]利用透明质酸水凝胶包裹凋亡小体作为防治材料,细胞实验中,将凋亡小体和人脐静脉内皮细胞混合培养来观察不同组别的网状结构情况,其中实验组的试管表现出更密集和更扩展的管状网,实验组的细胞增殖长度(17 115.0±565.6) μm显著高于对照组(12 146.0± 392.5) μm;透明质酸/凋亡小体组的妊娠率明显高于自然修复组和凋亡小体组;将其注射进小鼠急性子宫内膜损伤模型中,含凋亡小体透明质酸水凝胶的植入明显增加了子宫内膜厚度和子宫内膜腺体数量、减少了纤维化,并促进了子宫内膜修复和生育能力的恢复,组织学染色也证实了透明质酸/凋亡小体组的胎盘具有高度血管化结构。透明质酸水凝胶搭载细胞可明显降低宫腔粘连复发及促进子宫内膜修复,具有较好的前景。 干细胞疗法在宫腔中应用的优点是不存在免疫排斥、促进内膜再生的同时降低子宫内膜纤维化,缺点在于不能直接将干细胞用于组织伤口,需要一定的载体搭载包覆改善其存活时间和提高再生能力。对于临床治疗而言,更多的透明质酸水凝胶起着一个屏障作用。TAFTI等[58]募集了65例接受子宫间隔切除术的妇女(病例组34例、对照组31例),病例组注射透明质酸水凝胶,对照组注射等量生理盐水,结果显示干预后,对照组阿舍尔曼综合征的发生率高于病例组(P=0.012),干预结束时病例组仅有4例发生粘连复发,对照组12例发生粘连复发,足以证明在子宫腔注射透明质酸的情况下有可能预防子宫内膜损伤的Asherman综合征。一项长期且大量的Meta分析表明,透明质酸水凝胶可显著降低宫腔粘连分离术后患者宫腔粘连的发生率、提高妊娠率、促进子宫内膜修复和再生[59]。 传统透明质酸水凝胶的缺点在于无法提升其他性能,比如高效地促进组织修复、提升可降解性和提高妊娠率等,因此越来越多的人将水凝胶交联改性以提高其性能。 (2)交联透明质酸水凝胶:传统透明质酸水凝胶缺乏强度,容易发生永久性断裂,而且内部的结构简单,缺乏特殊的功能,难以适应多变的宫内情况,这很大程度上限制了它们的应用。交联透明质酸水凝胶是在透明质酸基础上采用物理/化学交联方法制备而成的水凝胶。物理交联制备的水凝胶也较为优异,而且制备条件较为温和;化学交联制备的水凝胶通常具有更强的机械性能和稳定性。近年来,通过物理/化学改性制备的交联透明质酸水凝胶可以提高其性能,取得更好的防粘连效果。 KIM等[60]利用制作的交联透明质酸水凝胶来包裹子宫内膜基质细胞,并将其应用于小鼠宫腔粘连模型,在2周内与对照组相比,水凝胶治疗组明显减少纤维组织的数量、增加子宫内膜厚度。通过3个胚层(Sox2、Nestin、HNF4α)的不同标记共同定位来评估成功植入的胚胎和胚胎的正常发育(n=37),再生的子宫内膜显示出功能恢复。当移植胚胎成功,经子宫内膜基质细胞负载的交联透明质酸水凝胶处理后,后代可以正常发育和活产。为了进一步探索治疗效果,WANG等[61]将间充质干细胞-水凝胶系统应用到恒河猴模型中,注射2个月后,子宫内膜基质细胞/交联透明质酸水凝胶组所有猴子月经恢复循环,子宫内膜的腺体数目明显大于单独交联透明质酸水凝胶组(P < 0.01),交联透明质酸水凝胶组移植后子宫内膜厚度无显著差异,而子宫内膜基质细胞/交联透明质酸水凝胶复合物移植后子宫内膜厚度显著增加(P < 0.01),与子宫内膜机械损伤前的正常水平相似,结果显示子宫内膜基质细胞/交联透明质酸水凝胶复合物移植在抗黏附特性和促进子宫内膜再生的双重修复作用方面具有显著优势;在细胞检测中,子宫内膜基质细胞/交联透明质酸水凝胶组细胞增殖明显优于其他组别,同时此组别纤维化程度明显降低。 交联透明质酸水凝胶不仅仅用于动物实验,LI等[62]制备了新型交联透明质酸水凝胶用于预防、延迟流产患者扩张和刮除后的宫腔粘连,征集300例患者进行试验,分为对照组和交联透明质酸水凝胶组,人数比为1∶1,交联透明质酸水凝胶凝胶组的宫内腔受累程度、粘连类型、月经模式、累积粘连评分均显著低于对照组(分别为P=0.000 7,P=0.008,P=0.001 2,P=0.000 6),中重度宫腔粘连女性比例显著低于对照组(P=0.000 2,相对风险为 0.062 5,95%CI值0.008 4-0.464 8),组间差异为 11.95%,结果显示使用新型交联透明质酸可明显降低宫腔粘连的复发。 透明质酸水凝胶不仅可以搭载基底细胞/间充质干细胞促进子宫内膜修复,还可以在实际临床上起到分隔腔部和降低子宫内膜纤维化的作用,适用于子宫内膜的修复,其中大量的透明质酸酶可以降解透明质酸水凝胶。使用不同交联方法可以限制干细胞等停留在损伤部位,为子宫内膜再生提供了理想的屏障性能。基于透明质酸水凝胶的诸多优势,不难看出它在宫腔粘连防治方面具有很好的前景[63]。 2.3.2 泊洛沙姆(Poloxamer)水凝胶 泊洛沙姆是一类水溶性非离子三嵌段共聚物,具有温和、无毒、无刺激性的性能,早已经过FDA批准并被纳入美国和欧洲药典[64],常用商品为泊洛沙姆127、泊洛沙姆407和泊洛沙姆188[65]。泊洛沙姆具有一种温度敏感特性,这使得可以通过此性质制备具有不同特性的热敏水凝胶,例如临界凝胶化浓度和生理条件下的凝胶化时间[66]。制备的泊洛沙姆水凝胶可以在体温条件下实现溶胶-凝胶转变。 雌激素具有增强抗纤维化的作用,防止粘连复发或术后粘连,并诱导宫腔粘连分离术后子宫内膜修复。近些年不断有人通过泊洛沙姆热敏水凝胶搭载雌二醇用来缓解宫腔粘连的复发。YAO等[67]利用芦荟提取物与泊洛沙姆溶液(质量分数17%)得到芦荟-泊洛沙姆溶液,随后利用溶剂蒸发法制备包覆了雌激素的去细胞子宫颗粒,将去细胞子宫颗粒加入到芦荟-泊洛沙姆溶液中,并将混合物进一步搅拌使颗粒均匀分散,水凝胶体系中雌二醇的质量浓度为0.2 mg/mL,体外模拟子宫发现,去细胞子宫颗粒可以显著延长水凝胶系统中雌激素的释放时间;在动物实验3 d后,接受雌激素-去细胞子宫颗粒-泊洛沙姆水凝胶的大鼠纤维化严重程度显著低于芦荟-泊洛沙姆组、市售雌激素凝胶组、雌激素单独组和宫腔粘连组;与3 d的结果相比,雌激素-去细胞子宫颗粒-泊洛沙姆水凝胶组治疗后7 d的纤维化面积进一步减少,仍然是所有治疗中宫腔粘连程度最低的,同样发现雌激素-去细胞子宫颗粒-泊洛沙姆可以下调转化生长因子β1的水平,且明显低于宫腔粘连组(P < 0.05)。以上结果不难得出,雌激素-去细胞子宫颗粒-泊洛沙姆水凝胶可有效促进子宫内膜再生、有效减缓子宫组织的纤维化,从而达到防止粘连形成的作用,防止子宫内膜再粘连。 ZHANG等[68]采用1-乙基3-氨基(3-二甲氨基丙基)碳二酰亚胺(EDC)和N-羟基琥珀酰亚胺(NHS)制备泊洛沙姆水凝胶,采用冷冻法制备了雌激素-泊洛沙姆水凝胶,包括不同量的泊洛沙姆水凝胶和17β-雌二醇,以实现用于宫腔粘连药物雌二醇的局部传递和缓释;在动物实验中,雌激素-泊洛沙姆水凝胶组的腺体数量明显增加,同假手术组情况相似;此外,雌激素-泊洛沙姆水凝胶处理后纤维化组织面积的比例明显降低。数据显示抑制粘连与通过激活2个下游信号通路PI3K/Akt和ERK1/2,与抑制内质网应激信号密切相关;腺体增生和抑制纤维化同时也和血管内皮生长因子有关。对于雌激素-泊洛沙姆水凝胶在防治宫腔粘连中的作用是不可置疑的。 以上研究表明,雌激素-泊洛沙姆水凝胶可能是治疗宫腔粘连一种很有前途的新方法。然而,对于临床前向临床的转化,还需要对治疗的长期效果进行更多的研究,但是不容置疑的是雌激素-泊洛沙姆水凝胶可有效降低宫腔粘连的复发。 2.3.3 合成/复合水凝胶 为了取得更好的子宫内膜修复效果和降低纤维化程度,越来越多的人将不同凝胶体系混合起来,制备成性能更为良好的合成/复合水凝胶,通常具有更好的生物相容性、优秀的下调炎症功能及延缓药物的释放等性能。 复合水凝胶是指将多种材料混合到一个水凝胶体系中以消除单一材料的缺陷,使复合材料获得全新的性能。例如,海藻酸钠水凝胶的细胞黏附性差、韧性差、降解缓慢,将其和明胶水凝胶进行交联得到具有优异的结构稳定性、韧性和细胞附着力的复合水凝胶。复合水凝胶的优势在于原料更为容易获取且制备简单。JI等[69]将30%明胶和4%海藻酸钠(体积比2∶1;37 ℃)混合制备3D打印油墨,利用3D打印技术制备混合物明胶-海藻酸钠水凝胶,打印完成后将形成的3D水凝胶支架立即浸泡在100 g/L氯化钙溶液中进行交联,利用复合水凝胶搭载人工诱导的间充质干细胞,在细胞检测中虽然只有少数间充质干细胞可以分化为子宫内膜相关细胞,但它们可能通过分泌或调节整合素αVβ3和白血病抑制因子等活性因子发挥更重要的作用;在宫腔粘连动物模型中,组织切片用Masson染色显示3D打印+间充质干细胞组的子宫内膜厚度-子宫壁厚度比更接近假手术组,证明3D打印的复合水凝胶支架可以帮助损伤子宫内膜的结构和功能恢复。结果表明,3D打印复合水凝胶可修复受损子宫内膜和恢复其支持胚胎的能力,并且降低子宫内膜纤维化程度。 FENG等[70]基于凝胶和胶原蛋白的生物相容性和生物降解性,合成了甲基丙烯酸明胶和甲基丙烯酸化胶原聚合物,使用3D打印后,甲基丙烯酸明胶/甲基丙烯酸化胶原水凝胶具有良好的结构保真度,采用光交联法制备了具有良好机械性能的支架,足以支撑其自身的质量和扭转。体外降解实验发现,甲基丙烯酸明胶/甲基丙烯酸化胶原水凝胶的降解率符合组织工程学要求,最终制备的水凝胶具有多孔的三维结构,提高了力学性能并延长了降解时间。用甲基丙烯酸明胶和甲基丙烯酸明胶/甲基丙烯酸化胶原培养人羊膜间充质干细胞,结果显示甲基丙烯酸明胶/甲基丙烯酸化胶原水凝胶组第7天的细胞增殖明显优于甲基丙烯酸明胶组,说明甲基丙烯酸化胶原的加入可以更好地支持细胞的增殖和生长,这与染色结果一致。体外实验结果表明,干细胞在孔状通道内壁表面更易实现附着和均匀扩散,并伴有细胞存活率高、增殖率正常和完整的细胞形态,并且在3D打印的甲基丙烯酸明胶/甲基丙烯酸化胶原水凝胶中,干细胞在体外连续释放至少7 d。随后动物模型宫腔粘连实验中,甲基丙烯酸明胶/甲基丙烯酸化胶原/人羊膜间充质干细胞水凝胶组的纤维化面积百分比明显低于模型组,且粘连程度明显更低。虽然3D打印技术仍面临着技术和材料上面的问题,但不可否认的是3D打印水凝胶可能为组织工程的开发研究提供一种可行和有效的途径,未来有望成为治疗宫腔粘连复发的高效方法。 合成水凝胶通常以聚乙二醇、聚乙烯醇、聚乳酸、聚羟基乙酸、聚乳酸-羟基乙酸共聚物等为代表的聚合物通过小分子单体或者合成高分子制备的一种材料,具有优秀的组织相容性和体内可降解性,主要应用为软组织工程,如心肌、血液、神经、皮肤以及软骨,还可作为腹腔内抗粘连剂[71]。WANG等[72]合成了一种聚乙二醇-b-聚苯丙氨酸/聚乙二醇水凝胶,细胞实验证实该水凝胶对细胞无毒性,且对于L929成纤维细胞具有明显的抑制作用,随着浓度和处理时间的延长,抑制效率均有所提高;在大鼠宫腔粘连模型中,发现该复合水凝胶组相比对照组腺体明显增多、子宫内膜厚度增加,且内膜损伤几乎完全愈合,妊娠第14天发现该复合水凝胶组别的腺体最多、纤维化程度更低。综合结果得出复合水凝胶有效抑制子宫纤维化,提高胚胎移植的成功率。 在天然基材料、天然聚合物以及合成聚合物的基础上,可以制备更为良好的生物相容性、生物降解性、机械性能、多孔性的水凝胶来防止宫腔粘连复发的同时促进内膜修复。关于水凝胶如何防止粘连复发的研究还需进一步探索,阐明水凝胶系统的作用机制以确定其修复效果。 2.3.4 其余类的水凝胶类材料 为了达到对宫腔粘连的防治和促进子宫内膜修复作用,许多人员设计了其他类的水凝胶用在防止宫腔粘连。聚(对二氧杂环己烷-l-苯丙氨酸)已被证实在体内通过不断释放l-苯丙氨酸来增加胞内Ca2+浓度和上调钙调磷酸酶表达,最终使得成纤维细胞凋亡加快、抑制纤维化[73]。WANG等[72]在聚(对二氧杂环己烷-l-苯丙氨酸)基础上合成制备了聚(乙二醇)-b-聚(L-苯基-丙氨酸),并在氢键作用下制备了聚(乙二醇)-b-聚(L-苯基-丙氨酸)/聚乙二醇水凝胶,体外测试该水凝胶对L929成纤维细胞的抑制情况,发现随着水凝胶浓度和处理时间的不断增长,抑制效果越来越明显;在小鼠子宫损伤模型中,聚(乙二醇)-b-聚(L-苯基-丙氨酸)/ 聚乙二醇水凝胶水凝胶处理组内膜厚度、腺体数目明显高于假手术组,同时术后14 d的胚胎植入率更高、防粘连效果更好。多种水凝胶类物质均表现出了良好的积极作用,包括降低纤维化、促进基底细胞再生、促进血管生成和提高妊娠率等(表2)。"
| [1] PEPPAS NA, HILT JZ, KHADEMHOSSEINI A, et al. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345-1360. [2] PEPPAS NA, MIKOS AG. Preparation methods and structure of hydrogels. Hydrogels in medicine and pharmacy, CRC press. 2019:1-26. [3] BRANNON-PEPPAS L. Preparation and characterization of crosslinked hydrophilic networks. Studies in polymer science, Elsevier, 1990:45-66. [4] WICHTERLE O, LIM D. Hydrophilic gels for biological use. Nature. 1960; 185(4706):117-118. [5] AHMED EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res. 2015;6(2):105-121. [6] SELIKTAR D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124-1128. [7] YANG J, ZHANG YS, YUE K, et al. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1-25. [8] PEñA B, LAUGHTER M, JETT S, et al. Injectable hydrogels for cardiac tissue engineering. Macromol Biosci. 2018;18(6):1800079. [9] XIN P, HAN S, HUANG J, et al. Natural okra-based hydrogel for chronic diabetic wound healing. Chin Chem Lett. 2022:108125. [10] LI Y, RODRIGUES J, TOMAS H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev. 2012; 41(6):2193-2221. [11] CORREA S, GROSSKOPF AK, LOPEZ HERNANDEZ H, et al. Translational applications of hydrogels. Chem Rev. 2021;121(18):11385-11457. [12] SABRY D, MOSTAFA A, MEKAWEY D, et al. An experimental model: intrauterine adhesion versus subendometrial fibrosis. Biomed Res. 2018;29(17):3311-3318. [13] DEANS R, VANCAILLIE T, LEDGER W, et al. Live birth rate and obstetric complications following the hysteroscopic management of intrauterine adhesions including Asherman syndrome. Hum Reprod. 2018;33(10):1847-1853. [14] DOROFTEI B, DABULEANU AM, ILIE OD, et al. Mini-review of the new therapeutic possibilities in Asherman syndrome—Where are we after one hundred and twenty-six years? Diagnostics. 2020;10(9):706. [15] YANG JH, CHEN CD, CHEN SU, et al. The influence of the location and extent of intrauterine adhesions on recurrence after hysteroscopic adhesiolysis. BJOG. 2016;123(4):618-623. [16] YU D, WONG YM, CHEONG Y, et al. Asherman syndrome—one century later. Fertil Steril. 2008;89(4):759-779. [17] WANG X, ZHOU C, ZHANG Y. Research, application and development of human amniotic epithelial cells in the field of obstetrics and gynecology. Chin J Tissue Eng Res. 2021;25(25):4070. [18] HUANG XW, LIN MM, ZHAO HQ, et al. A prospective randomized controlled trial comparing two different treatments of intrauterine adhesions. Reprod Biomed Online. 2020;40(6):835-841. [19] AAGL ELEVATING GYNECOLOGIC SURGERY. AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynaecological Endoscopy (ESGE). Gynecol Surg. 2017;14(1):6. [20] ZHU X, PéAULT B, YAN G, et al. Stem cells and endometrial regeneration: from basic research to clinical trial. Curr Stem Cell Res Ther. 2019;14(4): 293-304. [21] HEALY MW, SCHEXNAYDER B, CONNELL MT, et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215(3):267-275.e7. [22] ZHANG P, ZHAO C, ZHAO T, et al. Recent advances in bioinspired gel surfaces with superwettability and special adhesion. Adv Sci. 2019;6(18):1900996. [23] DE FRANCE KJ, XU F, HOARE T. Structured macroporous hydrogels: Progress, challenges, and opportunities. Adv Healthc Mater. 2018;7(1):1700927. [24] TANG Y, LIN S, YIN S, et al. In situ gas foaming based on magnesium particle degradation: A novel approach to fabricate injectable macroporous hydrogels. Biomaterials. 2020;232:119727. [25] JIANG T, WANG T, LI T, et al. Enhanced transdermal drug delivery by transfersome-embedded oligopeptide hydrogel for topical chemotherapy of melanoma. ACS Nano. 2018;12(10):9693-9701. [26] MA Y, HAN T, YANG Q, et al. Viscoelastic cell microenvironment: hydrogel‐based strategy for recapitulating dynamic ECM mechanics. Adv Funct Mater. 2021;31(24):2100848. [27] DE GROOT CJ, VAN LUYN MJ, VAN DIJK-WOLTHUIS WN, et al. In vitro biocompatibility of biodegradable dextran-based hydrogels tested with human fibroblasts. Biomaterials. 2001;22(11):1197-1203. [28] SLAUGHTER BV, KHURSHID SS, FISHER OZ, et al. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32-33):3307-3329. [29] TOWNSEND JM, BECK EC, GEHRKE SH, et al. Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog Polym Sci. 2019;91:126-140. [30] MARYCZ K, SMIESZEK A, TRYNDA J, et al. Nanocrystalline hydroxyapatite loaded with resveratrol in colloidal suspension improves viability, metabolic activity and mitochondrial potential in human adipose-derived mesenchymal stromal stem cells (hASCs). Polymers. 2019;11(1):92. [31] PIERSON E, ALTHOFF T, THOMAS D, et al. Daily, weekly, seasonal and menstrual cycles in women’s mood, behaviour and vital signs. Nat Hum Behav. 2021;5(6):716-725. [32] WANG Y, KANKALA RK, OU C, et al. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact Mater. 2022;9:198-220. [33] LIN Y, DONG S, ZHAO W, et al. Application of hydrogel-based delivery system in endometrial repair. ACS Appl Bio Mater. 2020;3(11):7278-7290. [34] DONG R, MA S, ZHAO X, et al. Recent progress of Bioinspired Hydrogel-based delivery system for endometrial repair. Front Bioeng Biotechnol. 2022;10:1013217. [35] LOPEZ-MARTINEZ S, RODRIGUEZ-EGUREN A, DE MIGUEL-GOMEZ L, et al. Bioengineered endometrial hydrogels with growth factors promote tissue regeneration and restore fertility in murine models. Acta Biomater. 2021;135:113-125. [36] PABUÇCU E G, KOVANCI E, ŞAHIN Ö, et al. New crosslinked hyaluronan gel, intrauterine device, or both for the prevention of intrauterine adhesions. JSLS. 2019;23(1):e2018.00108. [37] WEIMAR CH, MACKLON NS, POST UITERWEER ED, et al. The motile and invasive capacity of human endometrial stromal cells: implications for normal and impaired reproductive function. Hum Reprod Update. 2013; 19(5):542-557. [38] CHEN G, LIU L, SUN J, et al. Foxf2 and Smad6 co‐regulation of collagen 5A2 transcription is involved in the pathogenesis of intrauterine adhesion. J Cell Mol Med. 2020;24(5):2802-2818. [39] TRINH TT, NGUYEN KD, PHAM HV, et al. Effectiveness of hyaluronic acid gel and intrauterine devices in prevention of intrauterine adhesions after hysteroscopic adhesiolysis in infertile women. J Minim Invasive Gynecol. 2022;29(2):284-290. [40] ARUNG W, MEURISSE M, DETRY O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17(41): 4545-4553. [41] LEE WL, LIU CH, CHENG M, et al. Focus on the primary prevention of intrauterine adhesions: current concept and vision. Int J Mol Sci. 2021; 22(10):5175. [42] LV H, WU B, SONG J, et al. Hydrogel, a novel therapeutic and delivery strategy, in the treatment of intrauterine adhesions. J Mater Chem B. 2021; 9(33):6536-6552. [43] ZAN J, SHUAI Y, ZHANG J, et al. Hyaluronic acid encapsulated silver metal organic framework for the construction of a slow-controlled bifunctional nanostructure: Antibacterial and anti-inflammatory in intrauterine adhesion repair. Int J Biol Macromol. 2023;230:123361. [44] FALLACARA A, BALDINI E, MANFREDINI S, et al. Hyaluronic acid in the third millennium. Polymers (Basel). 2018;10(7):701. [45] CHENG S, PAN M, HU D, et al. Adhesive chitosan-based hydrogel assisted with photothermal antibacterial property to prompt mice infected skin wound healing. Chin Chem Lett. 2023:108276. [46] TAVIANATOU AG, CAON I, FRANCHI M, et al. Hyaluronan: molecular size‐dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019;286(15):2883-2908. [47] ZAMBONI F, VIEIRA S, REIS R L, et al. The potential of hyaluronic acid in immunoprotection and immunomodulation: chemistry, processing and function. Prog Mater Sci. 2018;97:97-122. [48] VASVANI S, KULKARNI P, RAWTANI D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int J Biol Macromol. 2020;151:1012-1029. [49] ZHOU W, HU Z, WEI J, et al. Quantum dots-hydrogel composites for biomedical applications. Chin Chem Lett. 2022;33(3):1245-1253. [50] WEISSMANN B. The transglycosylative action of testicular hyaluronidase. J Biol Chem. 1955;216(2):783-794. [51] GRAçA MF, MIGUEL SP, CABRAL CS, et al. Hyaluronic acid—Based wound dressings: A review. Carbohydr Polym. 2020;241:116364. [52] LIU Y, CAI J, LUO X, et al. Collagen scaffold with human umbilical cord mesenchymal stem cells remarkably improves intrauterine adhesions in a rat model. Gynecol Obstet Invest. 2020;85(3):267-276. [53] MEDINA CB, MEHROTRA P, ARANDJELOVIC S, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580(7801): 130-135. [54] VOLL RE, HERRMANN M, ROTH E A, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658): 350-351. [55] LIU D, KOU X, CHEN C, et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018;28(9):918-933. [56] LIU J, QIU X, LV Y, et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res Ther. 2020;11(1):507. [57] XIN L, WEI C, TONG X, et al. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: A therapy for intrauterine adhesions. Bioact Mater. 2022;12:107-119. [58] TAFTI SZG, JAVAHERI A, FIROOZABADI RD, et al. Role of hyaluronic acid intrauterine injection in the prevention of Asherman’s syndrome in women undergoing uterine septum resection: An RCT. Int J Reprod Biomed. 2021; 19(4):339-346. [59] ZHENG F, XIN X, HE F, et al. Meta-analysis on the use of hyaluronic acid gel to prevent intrauterine adhesion after intrauterine operations. Exp Ther Med. 2020;19(4):2672-2678. [60] KIM YY, PARK KH, KIM YJ, et al. Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater. 2019;89:139-151. [61] WANG L, YU C, CHANG T, et al. In situ repair abilities of human umbilical cord–derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion. Sci Adv. 2020;6(21):eaba6357. [62] LI X, WU L, ZHOU Y, et al. New crosslinked hyaluronan gel for the prevention of intrauterine adhesions after dilation and curettage in patients with delayed miscarriage: a prospective, multicenter, randomized, controlled trial. J Minim Invasive Gynecol. 2019;26(1):94-99. [63] CAI G, HOU Z, SUN W, et al. Recent developments in biomaterial-based hydrogel as the delivery system for repairing endometrial injury. Front Bioeng Biotechnol. 2022;10:894252. [64] ZARRINTAJ P, JOUYANDEH M, GANJALI MR, et al. Thermo-sensitive polymers in medicine: A review. Eur Polym J. 2019;117:402-423. [65] ZARRINTAJ P, RAMSEY JD, SAMADI A, et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020;110:37-67. [66] GIOFFREDI E, BOFFITO M, CALZONE S, et al. Pluronic F127 Hydrogel Characterization and Biofabrication in Cellularized Constructs for Tissue Engineering Applications. Procedia CIRP. 2016;49:125-132. [67] YAO Q, ZHENG YW, LAN QH, et al. Aloe/poloxamer hydrogel as an injectable β-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur J Pharm Sci. 2020;148:105316. [68] ZHANG SS, XIA WT, XU J, et al. Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17β-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int J Nanomedicine. 2017;12:5643. [69] JI W, HOU B, LIN W, et al. 3D Bioprinting a human iPSC-derived MSC-loaded scaffold for repair of the uterine endometrium. Acta Biomater. 2020;116: 268-284. [70] FENG M, HU S, QIN W, et al. Bioprinting of a Blue Light-Cross-Linked Biodegradable Hydrogel Encapsulating Amniotic Mesenchymal Stem Cells for Intrauterine Adhesion Prevention. ACS Omega. 2021;6(36):23067-23075. [71] OLIVA N, CONDE J, WANG K, et al. Designing hydrogels for on-demand therapy. Acc Chem Res. 2017;50(4):669-679. [72] WANG B, FENG C, DANG J, et al. Preparation of fibroblast suppressive poly (ethylene glycol)-b-poly (l-phenylalanine)/poly (ethylene glycol) hydrogel and its application in intrauterine fibrosis prevention. ACS Biomater Sci Eng. 2020;7(1):311-321. [73] WANG B, WEN A, FENG C, et al. The in vivo anti-fibrotic function of calcium sensitive receptor (CaSR) modulating poly (p-dioxanone-co-l-phenylalanine) prodrug. Acta Biomater. 2018;73:180-189. [74] LIU F, HU S, YANG H, et al. Hyaluronic acid hydrogel integrated with mesenchymal stem cell‐secretome to treat endometrial injury in a rat model of Asherman’s syndrome. Adv Healthc Mater. 2019;8(14):1900411. [75] LIN Y, DONG S, YE X, et al. Synergistic regenerative therapy of thin endometrium by human placenta-derived mesenchymal stem cells encapsulated within hyaluronic acid hydrogels. Stem Cell Res Ther. 2022; 13(1):66. [76] XU X, KONG DS, TIAN YP, et al. Autocross-linked hyaluronic acid gel and adipose-derived mesenchymal stem cell composites for the treatment intrauterine adhesions. Taiwan J Obstet Gynecol. 2021;60(6):1031-1037. [77] YANG H, WU S, FENG R, et al. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res Ther. 2017;8:267. [78] XU HL, XU J, SHEN BX, et al. Dual regulations of thermosensitive heparin–poloxamer hydrogel using ε-polylysine: bioadhesivity and controlled KGF release for enhancing wound healing of endometrial injury. ACS Appl Mater Interfaces. 2017;9(35):29580-29594. [79] XU HL, XU J, ZHANG SS, et al. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017;24(1):867-881. [80] XIE X, XU R, OUYANG H, et al. A mechanically robust and stable estradiol-loaded PHEMA-based hydrogel barrier for intrauterine adhesion treatment. J Mater Chem B. 2022;10(42):8684-8695. [81] GUAN CY, WANG F, ZHANG L, et al. Genetically engineered FGF1-sericin hydrogel material treats intrauterine adhesion and restores fertility in rat. Regen Biomater. 2022;9:rbac016. [82] WENBO Q, LIJIAN X, SHUANGDAN Z, et al. Controlled releasing of SDF-1α in chitosan-heparin hydrogel for endometrium injury healing in rat model. Int J Biol Macromol. 2020;143:163-172. [83] LIN J, WANG Z, HUANG J, et al. Microenvironment‐protected exosome‐hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small. 2021;17(11):2007235. [84] XIN L, LIN X, ZHOU F, et al. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020; 113:252-266. [85] CAO Y, SUN H, ZHU H, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192. [86] CIMMINO L, NEEL BG, AIFANTIS I. Vitamin C in stem cell reprogramming and cancer. Trends Cell Biol. 2018;28(9): 698-708. [87] KONCI R, CAMINSKY N, TULANDI T, et al. Supplements to conventional treatment after hysteroscopic lysis of intrauterine adhesions: a systematic review. J Obstet Gynaecol Can. 2020;42(8):984-1000. [88] REED SD, ZHOU X, ICHIKAWA L, et al. Intrauterine device-related uterine perforation incidence and risk (APEX-IUD): a large multisite cohort study. Lancet. 2022; 399(10341):2103-2112. [89] ABBOTT J, DEANS R. Accelerating the science after 125 years of treating intrauterine adhesions. J Minim Invasive Gynecol. 2021;28(2):151-152. |
| [1] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [2] | Bai Chen, Yang Wenqian, Meng Zhichao, Wang Yuze. Strategies for repairing injured anterior cruciate ligament and promoting graft healing [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1457-1463. |
| [3] | Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992. |
| [4] | Wang Wen, Zheng Pengpeng, Meng Haohao, Liu Hao, Yuan Changyong. Overexpression of Sema3A promotes osteogenic differentiation of dental pulp stem cells and MC3T3-E1 [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 993-999. |
| [5] | Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006. |
| [6] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [7] | Wang Shanshan, Shu Qing, Tian Jun. Physical factors promote osteogenic differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1083-1090. |
| [8] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [9] | Xu Canli, He Wenxing, Wang Lei, Wu Fangting, Wang Jiahui, Duan Xuelin, Zhao Tiejian, Zhao Bin, Zheng Yang. Bibliometric analysis of researches on liver organoids [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1099-1104. |
| [10] | Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123. |
| [11] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [12] | Sun Yukang, Song Lijuan, Wen Chunli, Ding Zhibin, Tian Hao, Ma Dong, Ma Cungen, Zhai Xiaoyan. Visualization analysis of stem cell therapy for myocardial infarction based on Web of Science in recent ten years [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1143-1148. |
| [13] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [14] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| [15] | Wang Wu, Fan Xiaolei, Xie Jie, Hu Yihe, Zeng Min. Hydroxyapatite-polyvinyl alcohol/collagen-chitosan-gelatin composite hydrogel for repairing rabbit osteochondral defect [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 682-689. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||