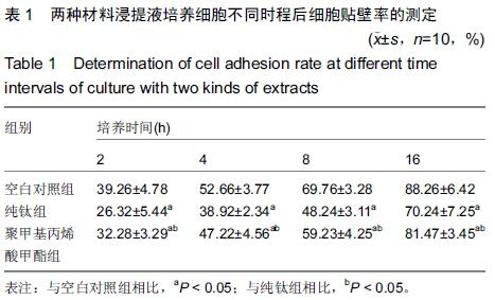
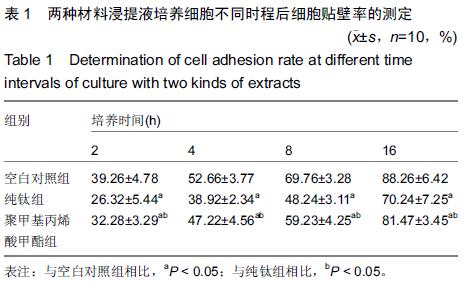
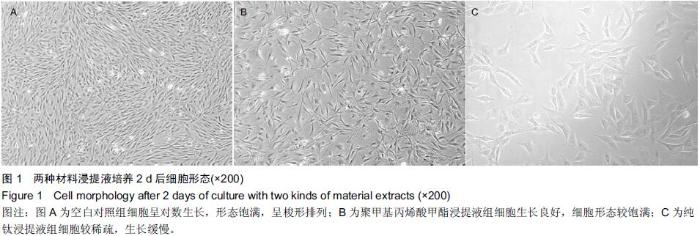
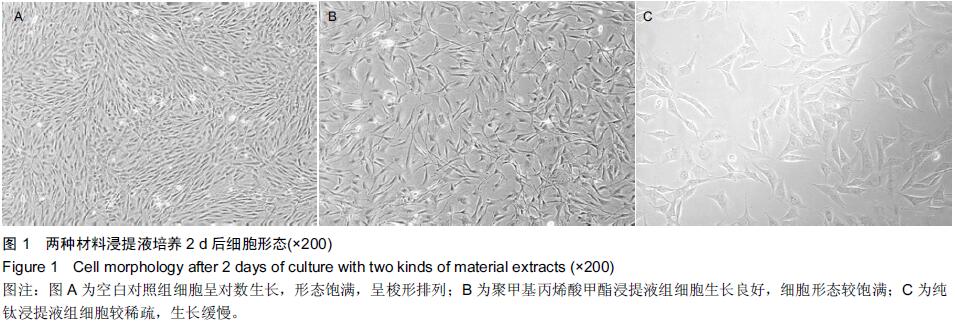
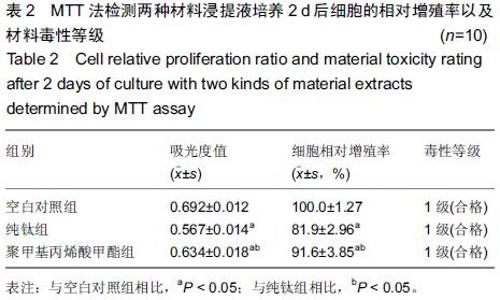
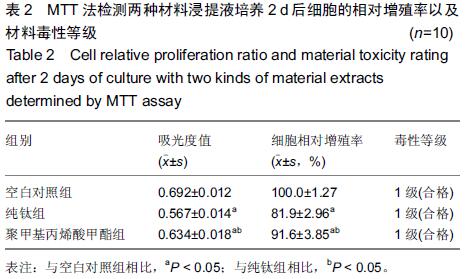
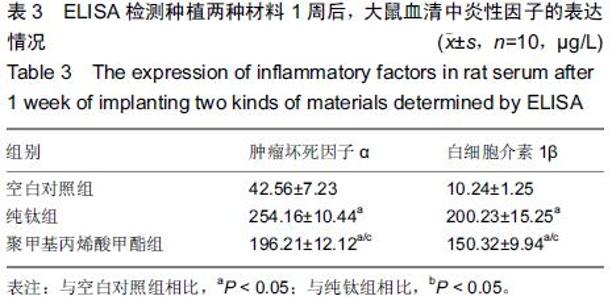
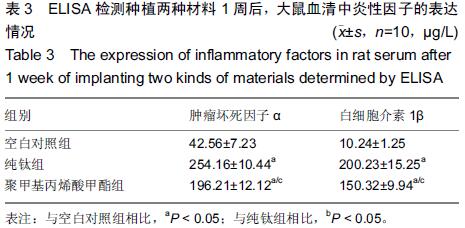
Chinese Journal of Tissue Engineering Research ›› 2015, Vol. 19 ›› Issue (47): 7613-7618.doi: 10.3969/j.issn.2095-4344.2015.47.012
Previous Articles Next Articles
Biocompatibility of polymethylmethacrylate as a polymer material for dental implants
Li Xiao-dong1, Li Xin-mei1, Sun Xiao-chen1, Sun Xiang2
- 1Department of Stomatology, Yan’an People’s Hospital, Yan’an 716000, Shaanxi Province, China; 2Department of Prosthodontics, School of Stomatology, the Fourth Military Medical University of PLA, Yan’an 710032, Shaanxi Province, China
-
Received:2015-10-13Online:2015-11-19Published:2015-11-19 -
Contact:Sun Xiang, Associate chief physician, M.D., Department of Prosthodontics, School of Stomatology, the Fourth Military Medical University of PLA, Yan’an 710032, Shaanxi Province, China -
About author:Li Xiao-dong, Master, Associate chief physician, Department of Stomatology, Yan’an People’s Hospital, Yan’an 716000, Shaanxi Province, China -
Supported by:the National Natural Science Foundation of China, No. 81371187; Science and Technology Program of Yan’an of China, No. 2011ks-05
Cite this article
Li Xiao-dong, Li Xin-mei, Sun Xiao-chen, Sun Xiang. Biocompatibility of polymethylmethacrylate as a polymer material for dental implants[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(47): 7613-7618.
share this article
| [1] Struemph JM, Chong AC, Wooley PH. Evaluation of different experience levels of orthopaedic residents effect on polymethylmethacrylate (PMMA) Bone Cement Mechanical Properties. Iowa Orthop J. 2015;35:193-198. [2] Carlsson E, Mestres G, Treerattrakoon K, et al. In Vitro and in vivo response to low-modulus pmma-based bone cement. Biomed Res Int. 2015; 2015:594284.
[3] Dan VN, Akhmedov BG, Krupochkin SN, et al. Use of polymethylmethacrylate in treatment of arteriovenous angiodysplasia with bone lesions. Angiol Sosud Khir. 2015; 21(3):153-158.
[4] Yu W, Xie H, Xin S, et al. Thermal Properties of Polymethyl Methacrylate Composite Containing Copper Nanoparticles. J Nanosci Nanotechnol. 2015;15(4):3121-3125.
[5] Rabiatul AR, Lokanathan Y, Rohaina CM, et al. Surface Modification of Electrospun Poly (Methylmethacrylate) (PMMA) Nanofibers for Development of In Vitro Respiratory Epithelium Model. J Biomater Sci Polym Ed. 2015:1-32.
[6] Greyling G, Pasch H. Multidetector thermal field-flow fractionation as a unique tool for the tacticity-based separation of poly(methylmethacrylate)-polystyrene block copolymer micelles.J Chromatogr A. 2015;1414:163-172.
[7] Sheafi EM, Tanner KE. Influence of test specimen fabrication method and cross-section configuration on tension-tension fatigue life ofPMMA bone cement. J Mech Behav Biomed Mater. 2015;51:380-387.
[8] Yadav NS, Saraf S, Mishra SK, et al. Effects of fluconazole, chlorhexidine gluconate, and silver-zinc zeolite on flexural strength of heat-curedpolymethyl methacrylate resin. J Nat Sci Biol Med. 2015;6(2):340-342.
[9] Arpornwichanop T, Polpanich D, Thiramanas R, et al. Enhanced antibacterial activity of NR latex gloves with raspberry-like PMMA-N,N,N-trimethyl chitosan particles. Int J Biol Macromol. 2015;81:151-158.
[10] Sorgini DB, Silva-Lovato CH, Muglia VA, et al. Adverse effects on pmma caused by mechanical and combined methods of denture cleansing. Braz Dent J. 2015;26(3): 292-296.
[11] Lim N, Pak Y, Kim JT, et al. A tunable sub-100 nm silicon nanopore array with an AAO membrane mask: reducing unwanted surface etching by introducing a PMMA interlayer. Nanoscale. 2015;7(32):13489-13494.
[12] Xu MM, Yan X, Deng XL. Study on interface compatibility and fracture resistance of polyglycidyl methacrylate pre-impregnated quartz fiber reinforced polymethyl methacrylate denture base resin. Beijing Da Xue Xue Bao. 2015;47(1):62-66.
[13] Kawaguchi T, Lassila LV, Sasaki H, et al. Effect of heat treatment of polymethyl methacrylate powder on mechanical properties of denture base resin. J Mech Behav Biomed Mater. 2014;39:73-78.
[14] Charland T, Hartwell GR, Hirschberg C, et al. An evaluation of setting time of mineral trioxide aggregate and EndoSequence root repair material in the presence of human blood and minimal essential media. J Endod. 2013; 39(8):1071-1072.
[15] Mahross HZ, Mohamed MD, Hassan AM et al.Effect of Cigarette Smoke on Surface Roughness of Different Denture Base Materials. J Clin Diagn Res. 2015;9(9):ZC39-ZC42.
[16] Basavarajappa S, Al-Kheraif AA, ElSharawy M, et al. Effect of solvent/disinfectant ethanol on the micro-surface structure and properties of multiphase denture base polymers. J Mech Behav Biomed Mater. 2015;54:1-7.
[17] Ayaz EA, Ba??? B, Turgut S. Effects of thermal cycling on surface roughness, hardness and flexural strength of polymethylmethacrylate and polyamide denture base resins. J Appl Biomater Funct Mater. 2015;13(3):e280-e286.
[18] Hazari P, Bhoyar A, Mishra SK, et al. A comparison of masticatory performance and efficiency of complete dentures made with high impact and flexible resins: a pilot study. J Clin Diagn Res. 2015;9(6):ZC29-ZC34.
[19] Mori K, Tsuji M, Ueda T, et al. Color and gloss evaluation of titanium dioxide coating for acrylic resin denture base. J Prosthodont Res. 2015;59(4):249-253.
[20] Panariello BH, Izumida FE, Moffa EB, et al. Effects of short-term immersion and brushing with different denture cleansers on the roughness, hardness, and color of two types of acrylic resin. Am J Dent. 2015;28(3):150-156.
[21] Sorgini DB, da Silva-Lovato CH, Muglia VA, et al. Adverse effects on PMMA caused by mechanical and combined methods of denture cleansing. Braz Dent J. 2015;26(3): 292-296.
[22] Khanna A, Bhatnagar VM, Karani JT, et al. A Comparative evaluation of shear bond strength between two commercially available heat cured resilient liners and denture base resin with different surface treatments. J Clin Diagn Res. 2015;9(5): ZC30-ZC34.
[23] Murthy HB, Shaik S, Sachdeva H, et al. Effect of reinforcement using stainless steel mesh, glass fibers, and polyethylene on the impact strength of heat cure denture base resin: an in vitro study. J Int Oral Health. 2015;7(6):71-79.
[24] Okayama S, Suzuki Y, Shimpo H, et al. Fixation of magnet assembly to denture base using alternative resins. Dent Mater J. 2015;34(3):364-370.
[25] Kamonwanon P, Yodmongkol S, Chantarachindawong R, et al. Wear resistance of a modified polymethyl methacrylate artificial tooth compared to five commercially available artificial tooth materials. J Prosthet Dent. 2015;114(2): 286-292.
[26] Matsuo H, Suenaga H, Takahashi M, et al. Deterioration of polymethyl methacrylate dentures in the oral cavity. Dent Mater J. 2015;34(2):234-239.
[27] Xu MM, Yan X, Deng XL. Study on interface compatibility and fracture resistance of polyglycidyl methacrylate pre-impregnated quartz fiber reinforced polymethyl methacrylate denture base resin. Beijing Da Xue Xue Bao. 2015;47(1):62-66.
[28] Ayaz EA, Durkan R, Koroglu A, et al. Comparative effect of different polymerization techniques on residual monomer and hardness properties ofPMMA-based denture resins. J Appl Biomater Funct Mater. 2014;12(3):228-233.
[29] Kawaguchi T, Lassila LV, Sasaki H, et al. Effect of heat treatment of polymethyl methacrylate powder on mechanical properties of denture base resin. J Mech Behav Biomed Mater. 2014;39:73-78.
[30] Akin H, Kirmali O, Tugut F, et al. Effects of different surface treatments on the bond strength of acrylic denture teeth to polymethylmethacrylatedenture base material. Photomed Laser Surg. 2014;32(9):512-516.
[31] Thoma DS, Brandenberg F, Fehmer V, et al. Randomized Controlled Clinical Trial of All-Ceramic Single Tooth Implant Reconstructions Using Modified Zirconia Abutments: Radiographic and Prosthetic Results at 1 Year of Loading. Clin Implant Dent Relat Res. 2015.
[32] Taylor TD, Klotz MW, Lawton RA. Titanium tattooing associated with zirconia implant abutments: a clinical report of two cases. Int J Oral Maxillofac Implants. 2014;29(4): 958-960.
[33] Held U, Rohner D, Rothamel D. Early loading of hydrophilic titanium implants inserted in low-mineralized (D3 and D4) bone: one year results of a prospective clinical trial. Head Face Med. 2013;9:37.
[34] Zembic A, Bösch A, Jung RE, et al. Five-year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single-implant crowns in canine and posterior regions. Clin Oral Implants Res. 2013; 24(4):384-390.
[35] Gong H, Wang L, Zheng D, et al. The potential application of functionally graded material for proximal femoral nail antirotation device. Med Hypotheses. 2012;79(3):415-417.
[36] Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review. Clin Oral Implants Res. 2009;20 Suppl 4:113-123.
[37] Jung SW, Son MK, Chung CH, et al. Abrasion of abutment screw coated with TiN. J Adv Prosthodont. 2009;1(2): 102-106.
[38] Lekholm U, Gröndahl K, Jemt T. Outcome of oral implant treatment in partially edentulous jaws followed 20 years in clinical function. Clin Implant Dent Relat Res. 2006;8(4): 178-186.
[39] Wang ML, Tuli R, Manner PA, et al. Direct and indirect induction of apoptosis in human mesenchymal stem cells in response to titanium particles. J Orthop Res. 2003;21(4): 697-707.
[40] Engelke W, Schwarzwäller W, Behnsen A, et al. Subantroscopic laterobasal sinus floor augmentation (SALSA): an up-to-5-year clinical study. Int J Oral Maxillofac Implants. 2003;18(1):135-143.
[41] Artzi Z, Dreiangel A. A screw lock for single-tooth implant superstructures. J Am Dent Assoc. 1999;130(5):677-682.
[42] Knabe C, Schendel KU. The use of implant-supported titanium prostheses for treatment of periodontally compromised patients including functional and orthodontic therapy. Report of 2 cases. Clin Oral Implants Res. 1997;8(4): 332-338.
|
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Li Xingping, Xiao Dongqin, Zhao Qiao, Chen Shuo, Bai Yiguang, Liu Kang, Feng Gang, Duan Ke. Preparation and properties of copper-loaded antibacterial functional film on titanium surface [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 553-557. |
| [5] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [6] | Shi Xiaoxiu, Mao Shilong, Liu Yang, Ma Xingshuang, Luo Yanfeng. Comparison of tantalum and titanium (alloy) as orthopedic materials: physical and chemical indexes, antibacterial and osteogenic ability [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 593-599. |
| [7] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [8] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [9] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [10] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [11] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [12] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [13] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [14] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [15] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||