Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (15): 2302-2306.doi: 10.12307/2024.402
Previous Articles Next Articles
Effect of nano-modified titanium surface with alkali heat treatment on early adhesion and growth of osteoblasts
Gao Yan1, Lin Xi1, Liu Ying2
- 1Implant Center, 2Department of Dentistry and Endodontics, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou 510280, Guangdong Province, China
-
Received:2023-05-13Accepted:2023-07-08Online:2024-05-28Published:2023-09-19 -
Contact:Liu Ying, MD, Associate chief physician, Department of Dentistry and Endodontics, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou 510280, Guangdong Province, China -
About author:Gao Yan, MD, Associate chief physician, Implant Center, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou 510280, Guangdong Province, China -
Supported by:Natural Science Foundation of Guangdong Province, No. 2018A030310439 (to GY); Guangdong Medical Science and Technology Research Fund Project, No. A2021480 (to GY); Guangdong Medical Science and Technology Research Fund Project, No. B2021061 (to LX)
CLC Number:
Cite this article
Gao Yan, Lin Xi, Liu Ying. Effect of nano-modified titanium surface with alkali heat treatment on early adhesion and growth of osteoblasts[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2302-2306.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
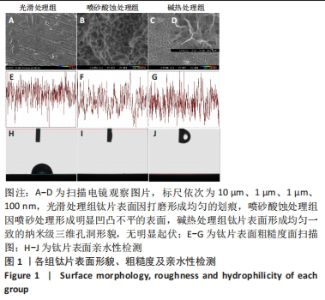
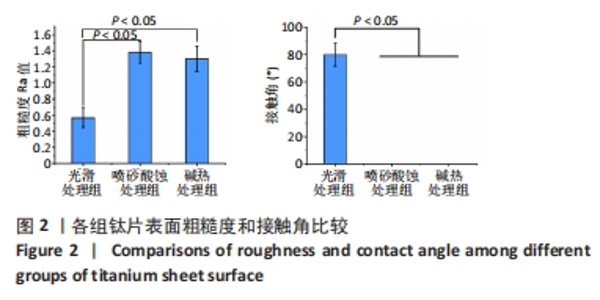
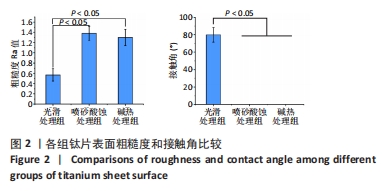
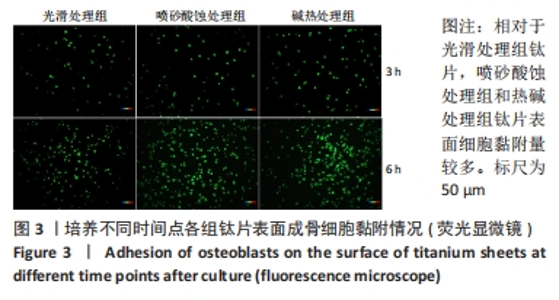
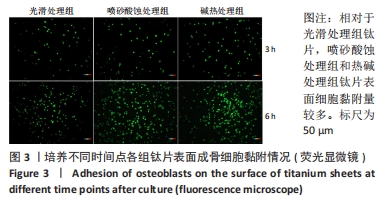
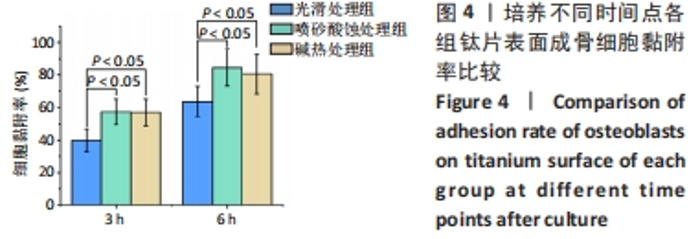
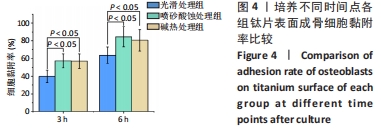
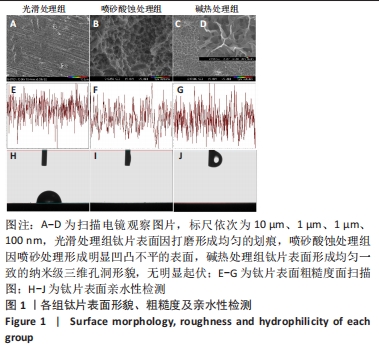
2.1 各组钛片表面形貌、粗糙度及亲水性检测结果 各组钛片表面的扫描电镜观察结果见图1A-D,光滑处理组钛片表面因打磨形成带有均匀的划痕,喷砂酸蚀处理组因喷砂处理形成明显凹凸不平的表面,碱热处理组钛片表面形成均匀一致的纳米级三维孔洞形貌,无明显起伏。 图1E-G为3组钛片表面粗糙度面扫描图;图1H-J为3组钛片表面亲水性检测图片,当水滴接触钛片表面时,喷砂酸蚀处理组和碱热处理组钛片表面水滴迅速散开,而光滑处理组水滴与钛片表面成一定角度。比较3组钛片表面的粗糙度值与接触角,结果显示:喷砂酸蚀处理组、碱热处理组钛片表面的粗糙度值大于光滑组(P < 0.05),接触角低于光滑处理组(P < 0.05);喷砂酸蚀处理组、碱热处理组钛片表面的粗糙度值与接触角比较差异均无显著性意义(P > 0.05),见图2。"
| [1] DANIEL B, LARS S, HUGO DB. Modern implant dentistry based on osseointegr- ation: 50 years of progress, current trends and open questions. Periodontol 2000. 2017;73(1):7-21. [2] 车振家,朱正清,朱礼伟,等.钛植入物表面生物化学改性对骨整合的影响[J].中国组织工程研究,2022,26(16):2576-2583. [3] SCULEAN A, GRUBER R, BOSSHARDT DD. Soft tissue wound healing around teeth and dental implants. J Clin Periodontol. 2014;41 Suppl 15:S6-22. [4] HASEGAWA M, SARUTA J, HIROTA M, et al. A newly created meso-, micro-, and nano-scale rough titanium surface promotes bone-implant integration. Int J Mol Sci. 2020;21(3):783. [5] 王旻,姜楠,祝颂松.新型钛表面微纳米共存梯度仿生结构对骨髓间充质细胞黏附、增殖及成骨分化的影响[J].口腔疾病防治,2021,29(4):226-233. [6] PINTO TS, MARTINS BR, FERREIRA MR, et al. Nanohydroxyapatite-Blasted Bioactive Surface Drives Shear-Stressed Endothelial Cell Growth and Angiogenesis. Biomed Res Int. 2022;2022:1433221. [7] WANG L, XU C, MENG K, et al. Biomimetic Hydroxyapatite Composite Coatings with a Variable Morphology Mediated by Silk Fibroin and Its Derived Peptides Enhance the Bioactivity on Titanium. ACS Biomater Sci Eng. 2023;9(1):165-181. [8] LÓPEZ-VALVERDE N, ARAGONESES J, LÓPEZ-VALVERDE A, et al. Effectiveness of biomolecule-based bioactive surfaces, on os-seointegration of titanium dental implants: A systematic review and meta-analysis of in vivo studies. Front Bioeng Biotechnol. 2022;10:986112. [9] WU J, JIANG S, XIE W, et al. Surface modification of the Ti surface with nanoscale bio-MOF-1 for improving biocompatibility and osteointegration in vitro and in vivo. J Mater Chem B. 2022;10(41):8535-8548. [10] CHEN M, WANG D, LI M, et al. Nanocatalytic Biofunctional MOF Coating on Titanium Implants Promotes Osteoporotic Bone Regeneration through Cooperative Pro-osteoblastogenesis MSC Reprogramming. ACS Nano. 2022;16(9):15397-15412. [11] LI Y, CHEN G, HE Y, et al. Selenomethionine-Modified Polyethylenimine-Based Nanoparticles Loaded with miR-132-3p Inhibitor-Biofunctionalized Titanium Implants for Improved Osteointegration. ACS Biomater Sci Eng. 2021;7(10):4933-4945. [12] SUN T, HUANG J, ZHANG W, et al. Simvastatin-hydroxyapatite coatings prevent biofilm formation and improve bone formation in implant-associated infections. Bioact Mater. 2022;21:44-56. [13] ZHU LS, LUO D, LIU Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int J Oral Sci. 2020;12(1):6. [14] DIOMEDE F, MARCONI GD, FONTICOLI L, et al. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int J Mol Sci. 2020;21(9):3242. [15] MA L, LI G, LEI J, et al. Nanotopography Sequentially Mediates Human Mesenchymal Stem Cell-Derived Small Extracellular Vesicles for Enhancing Osteogenesis. ACS Nano. 2022;16(1):415-430. [16] XUE Y, ZHANG L, LIU F, et al. Surface Bandgap Engineering of Nanostructured Implants for Rapid Photothermal Ion Therapy of Bone Defects. Adv Healthc Mater. 2022;11(22):e2200998. [17] KIM HM, MIYAJI F, KOKUBO T, et al. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J Biomed Mater Res. 1996;32(3):409-417. [18] Baek HL, YOUNG DK, JI HS, et al. Surface modification by alkali and heat treatments in titanium alloys. J Biomed Mater Res. 2002;61(3):466-473. [19] TADASHI K, SEIJI Y. Groeth of novel ceramic layers on metals via chemical and heat treatments for inducing various biological functions. Front Bioeng Biotechnol. 2015;3:176. [20] WANG XJ, LI YC, LIN JG, et al. Effect of heat-treatment atmosphere on the bond strength of apatite layer on Ti substrate. Dent Mater. 2008;24(11):1549-1555. [21] ZHOU Y, WANG YB, ZHANG EW, et al. Alkali-heat treatment of a low modulus biomedical Ti-27NB alloy. Biomed Mater. 2009;4(4):044108. [22] ZHAO LZ, HU LS, HUO KF, et al. Mechanism of cell repellence on quasi-aligned nanowires arrays on Ti alloy. Biomaterials. 2010;31(32):8341-8349. [23] DING XL, YANG XQ, ZHOU L, et al. Titanate nanowires scaffolds decorated with anatase nanocrystals show good protein adsorption and low cell adhesion capacity. Int J Nanomedicine. 2013;8:569-579. [24] SticH T, ALAGBOSO F, KŘENEK T, et al. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng Transl Med. 2021;7(1):e10239. [25] Li S, Ni J, Liu X, et al. Surface characteristics and biocompatibility of sandblasted and acid-etched titanium surface modified by ultraviolet irradiation: an in vitro study. J Biomed Mater Res B Appl Biomater. 2012;100(6):1587-1598. [26] DADFARNIA M, NOVAK P, AHN DC, et al. Recent advances in the study of structural materials compatibility with hydrogen. Adv Mater. 2010;22(10):1128-1135. [27] HU P, GAO Q, ZHENG H, et al. The Role and Activation Mechanism of TAZ in Hierarchical Microgroove/Nanopore Topography-Mediated Regulation of Stem Cell Differentiation. Int J Nanomedicine. 2021;16:1021-1036. [28] MA L, KE W, LIAO Z, et al. Small extracellular vesicles with nanomorphology memory promote osteogenesis. Bioact Mater. 2022;17:425-438. [29] YU X, XU R, ZHANG Z. Different Cell and Tissue Behavior of Micro-/Nano-Tubes and Micro-/Nano-Nets Topographies on Selective Laser Melting Titanium to Enhance Osseointegration. Int J Nanomedicine. 2021;16:3329-3342. [30] FUJIBAYASHI S, NAKAMURA T, NISHIGUCHI S, et al. Bioactive titanium: effect of sodium removal on the bone-bonding ability of bioactive titanium prepared by alkali and heat treatment. J Biomed Mater Res. 2001;56(4):562-570. [31] KIM HM, MIYAJI F, KOKUBO T, et al. Graded surface structure of bioactive titanium prepared by chemical treatment. J Biomed Mater Res. 1999;45(2):100-107. [32] BOERCKER JE, ENACHE-POMMER E, AYDIL ES. Growth mechanism of titanium dioxide nanowires for dye-sensitized solar cells. Nanotechnology. 2008;19(9): 095604. [33] WANG F, DAI HX, DENG JG, et al. Manganese oxides with rod-, wire-, tube-, and flower-like morphologies: highly effective catalysts for the removal of toluene. Environ Sci Technol. 2012;46(7):4034-4041. [34] LIU W, LIANG L, LIU B, et al. The response of macrophages and their osteogenic potential modulated by micro/nano-structured Ti surfaces. Colloids Surf B Biointerfaces. 2021;205:111848. [35] MASAMOTO K, FUJIBAYASHI S, YAMAGUCHI S, et al. Bioactivity and antibacterial activity of strontium and silver ion releasing titanium. J Biomed Mater Res B Appl Biomater. 2021;109(2):238-245. [36] OKUZU Y, FUJIBAYASHI S, YAMAGUCHI S, et al. In vitro study of antibacterial and osteogenic activity of titanium metal releasing strontium and silver ions. J Biomater Appl. 2021;35(6):670-680. [37] DOYMUS B, KEREM G, YAZGAN KARATAS A, et al. A functional coating to enhance antibacterial and bioactivity properties of titanium implants and its performance in vitro. J Biomater Appl. 2021;35(6):655-669. [38] JEONG SJ, JEONG MJ. Effect of Thymosin beta4 on the Differentiation and Mineralization of MC3T3-E1 Cell on a Titanium Surface. J Nanosci Nanotechnol. 2016;16(2):1979-1983. [39] GAO Q, HOU Y, LI Z, et al. mTORC2 regulates hierarchical micro/nano topography-induced osteogenic differentiation via promoting cell adhesion and cytoskeletal polymerization. J Cell Mol Med. 2021;25(14):6695-6708. [40] WANG H, XU Q, HU H, et al. The Fabrication and Function of Strontium-modified Hierarchical Micro/Nano Titanium Implant. Int J Nanomedicine. 2020;15:8983-8998. [41] ALBERTINI M, FERNANDEZ-YAGUE M, LÁZARO P, et al. Advances in surfaces and osseointegration in implantology. Biomimetic surfaces. Med Oral Patol Oral Cir Bucal. 2015;20(3):e316-325. [42] DIAS CORPA TARDELLI J, LIMA DA COSTA VALENTE M, THEODORO DE OLIVEIRA T, et al. Influence of chemical composition on cell viability on titanium surfaces: A systematic review. J Prosthet Dent. 2021;125(3):421-425. [43] LIU J, RUAN J, YIN J, et al. Fabrication of multilevel porous structure networks on Nb-Ta-Ti alloy scaffolds and the effects of surface characteristics on behaviors of MC3T3-E1 cells. Biomed Mater. 2022;17(6). doi: 10.1088/1748-605X/ac9ffd. [44] SHICHMAN I, OAKLEY C, WILLEMS JH, et al. Novel metaphyseal porous titanium cones allow favorable outcomes in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2023;143(3):1537-1547. [45] ZHANG Y, SUN N, ZHU M, et al. The contribution of pore size and porosity of 3D printed porous titanium scaffolds to osteogenesis. Biomater Adv. 2022;133:112651. [46] DAVIES JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003; 67(8):932-949. [47] PULEO DA, NANCI A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20(23-24):2311-2321. [48] HORI N, UENO T, SUZUKI T, et al. Ultraviolet light treatment for the restoration of age-related degradation of titanium bioactivity. Int J Oral Maxillofac Implants. 2010;25(1):49-62. [49] MIYAUCHI T, YAMADA M, YAMAMOTO A, et al. The enhanced characteristics of osteoblast adhesion to photofunctionalized nanoscale TiO2 layers on biomaterials surfaces. Biomaterials. 2010;31(14):3827-3839. [50] TabUCHI M, HAMAJIMA K, TANAKA M, et al. UV Light-Generated Superhydrophilicity of a Titanium Surface Enhances the Transfer, Diffusion and Adsorption of Osteogenic Factors from a Collagen Sponge. Int J Mol Sci. 2021; 22(13):6811. |
| [1] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [2] | Bai Chen, Yang Wenqian, Meng Zhichao, Wang Yuze. Strategies for repairing injured anterior cruciate ligament and promoting graft healing [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1457-1463. |
| [3] | Wang Wen, Zheng Pengpeng, Meng Haohao, Liu Hao, Yuan Changyong. Overexpression of Sema3A promotes osteogenic differentiation of dental pulp stem cells and MC3T3-E1 [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 993-999. |
| [4] | Wei Yuanxun, Chen Feng, Lin Zonghan, Zhang Chi, Pan Chengzhen, Wei Zongbo. The mechanism of Notch signaling pathway in osteoporosis and its prevention and treatment with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 587-593. |
| [5] | Zhu Zhiqi, Yuan Sijie, Zhang Zilin, Ji Shijie, Meng Mingsong, Yan Anming, Han Jing. Mechanism underlying the effect of Liuwei Dihuang Pill on osteolysis and osteogenesis induced by titanium particles [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 392-397. |
| [6] | Gao Xueyu, Zhang Wentao, Sun Tianze, Zhang Jing, Li Zhonghai. Application of metal ions in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 439-444. |
| [7] | Yang Jie, Hu Haolei, Li Shuo, Yue Wei, Xu Tao, Li Yi. Application of bio-inks for 3D printing in tissue repair and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 445-451. |
| [8] | Chen Junyan, Meng Qingqi, Li Siming. Cartilage targeting function in the drug delivery system by intra-articular injection for the treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 458-463. |
| [9] | Kong Xiangyu, Wang Xing, Pei Zhiwei, Chang Jiale, Li Siqin, Hao Ting, He Wanxiong, Zhang Baoxin, Jia Yanfei. Biological scaffold materials and printing technology for repairing bone defects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 479-485. |
| [10] | Chen Xiangshan, Liu Hua, Sun Weikang, Li Huanan. Mechanism of m6A methylation regulating bone metabolism for prevention and treatment of osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(28): 4572-4577. |
| [11] | Zhang Shudong, Huang Yilin, Yao Qi. Punicalagin treats postmenopausal osteoporosis by promoting osteogenesis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(26): 4101-4105. |
| [12] | Li Jiale, Luo Dasheng, Zheng Liujie, Liu Wei, Yao Yunfeng. Human osteoarthritic chondrocytes up-regulate the expression of osteoprotegerin in osteoblasts via the Indian hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(26): 4194-4201. |
| [13] | Yang Qipei, Chen Feng, Cui Wei, Zhang Chi, Wu Ruiqi, Song Zhenheng, Meng Xin. Signaling pathways related to kaempferol active monomers in the treatment of osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(26): 4242-4249. |
| [14] | Yu Yangyi, Lian Qiang, Wu Jianqun, Zhang Xuan, Ren Jinke, Li Guangheng. Cell-of-origin for heterotopic ossification induced by bone morphogenetic protein 4 in skeletal muscle [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4034-4040. |
| [15] | Luo Peng, Wang Yi, Wang Ansu, Dang Yi, Ma Yaping, Zhang Yi, Wang Xin. Role and mechanism of interleukin-8 in bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(24): 3910-3914. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||