Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (1): 130-134.doi: 10.12307/2022.022
Previous Articles Next Articles
Effect of mesenchymal stem cells on myocardial ischemia-reperfusion injury
Zhao Xu1, Mao Xin2, Li Chuntian1, Wang Feng3
- 1Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 3The Second Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2021-01-25Revised:2021-01-29Accepted:2021-03-04Online:2022-01-08Published:2021-10-25 -
Contact:Wang Feng, MD, Associate chief physician, The Second Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Zhao Xu, Master candidate, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 81660074 (to WF)
CLC Number:
Cite this article
Zhao Xu, Mao Xin, Li Chuntian, Wang Feng. Effect of mesenchymal stem cells on myocardial ischemia-reperfusion injury[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 130-134.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
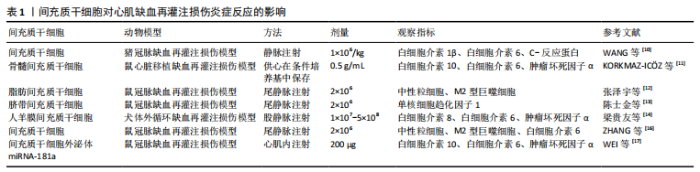
2.1 心肌缺血再灌注损伤中炎症损伤与间充质干细移植治疗 当前研究证实了过度的炎性反应是心肌缺血再灌注损伤的主要机制之一[2-3],如心肌组织中大量白细胞浸润、心肌细胞水肿、大量炎性细胞因子的释放,造成心肌细胞凋亡及组织坏死。已有报道指出炎症反应是缺血性心脏疾病、体外循环术后心功能障碍和死亡的重要原因之一[4]。近年来多项研究证实,心肌缺血再灌注损伤与炎症反应密切相关,炎症反应程度直接关系着损伤心肌的恢复情况,并且在早期治疗干预炎症反应,效果会更为理想[5-7]。HONG等[8]研究中发现,在大鼠心脏缺血再灌注损伤模型中,物质P可介导骨髓间充质干细胞更有效减轻心肌缺血再灌注损伤的早期炎症反应,并且物质P可促进骨髓间充质干细胞动员归巢,有效改善了心肌缺血再灌注损伤及急性心肌梗死。 目前研究指出炎症因子及免疫细胞水平的高低直接与心脏缺血再灌注后心脏功能及心肌细胞凋亡程度相关,因此调控炎症因子及免疫细胞对减轻炎症损伤起到了非常重要的作用[9]。WANG等[10]研究发现在猪心肌缺血再灌注损伤模型中,注射间充质干细胞可通过减少白细胞介素1β、白细胞介素6、C-反应蛋白等炎症因子水平及心肌中自然杀伤细胞数量,减轻炎症损伤及炎症后期纤维化形成的微血管阻塞。近年来大量研究证实了不同源性间充质干细胞对心肌缺血再灌注损伤炎症因子及免疫细胞的作用也略有差异。KORKMAZ-IC?Z等[11]研究发现在骨髓来源间充质干细胞条件培养基中保存的供心移植后收缩功能及舒张功能明显改善,其炎症细胞因子水平下调。张泽宇等[12]研究发现脂肪间充质干细胞移植后炎症因子、中性粒细胞降低,大鼠心肌缺血再灌注后炎症损伤减轻,心肌梗死面积减小,延缓心室重构,改善心脏功能。陈士金等[13]研究发现脐带间充质干细胞可降低单核细胞趋化因子1表达,提高组织中血管内皮生长因子表达,说明其能够抑制炎症反应发生并促进损伤组织血管重建。梁贵友等[14]在人羊膜间充质干细胞移植治疗犬心肌缺血再灌注损伤研究中,发现人羊膜间充质干细胞移植可有效保护心肌细胞,改善心脏功能,降低心肌损伤特异性蛋白水平,降低血浆炎症因子白细胞介素8和肿瘤坏死因子α水平,提高血浆抗炎因子白细胞介素10水平。作者前期研究中发现,将TIPE2基因修饰的人羊膜间充质干细胞与缺血再灌注损伤的心肌细胞共培养,在免疫调节及抑制炎症方面具有更好的作用[15],这也为后期深入研究基因修饰的间充质干细胞作用机制提供一种思路。 在间充质干细胞干预心肌缺血再灌注损伤炎症免疫反应的研究中,ZHANG等[16]研究发现,胞葬作用是缓解中性粒细胞介导的心肌缺血再灌注损伤早期免疫反应的关键。间充质干细胞输注可增强M2型巨噬细胞诱导的中性粒细胞胞葬作用,有效减轻大鼠心肌缺血再灌注损伤,改善大鼠心功能。SHIN等[7]研究发现,间充质干细胞可有效减少心肌组织中免疫细胞浸润,并通过激活CD73活性增加心肌缺血再灌注损伤后心肌腺苷的有效性,通过超声心动图发现CD73活性对心肌缺血再灌注损伤后心功能保护至关重要,表明间充质干细胞介导CD73将磷酸腺苷转化为腺苷,对心肌缺血再灌注损伤具有重要的抗炎作用。这两项研究都是间充质干细胞通过减少免疫细胞减轻心肌缺血再灌注损伤,但机制却不相同,间充质干细胞增强、极化巨噬细胞诱导免疫调节作用目前也是学界研究热点,包括深入研究间充质干细胞外泌体及其基因修饰在免疫诱导、免疫耐受等方面的影响。 目前关于间充质干细胞外泌体的研究不计其数。研究发现,间充质干细胞来源外泌体miRNA-181a对小鼠心肌缺血再灌注损伤有较强的治疗作用,展示了miRNA-181a在炎症损伤方面的调控潜力[17]。不同间充质干细胞对心肌缺血再灌注损伤炎症反应的影响,见表1。 "
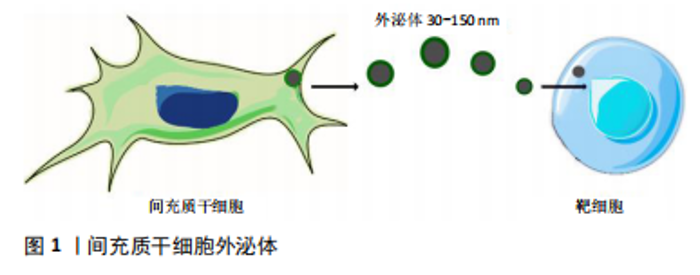
心肌缺血再灌注损伤的炎症反应对于清除坏死心肌组织是必要的,但再灌注本身却加重了心肌炎症反应,导致心肌组织血运重建后炎症反应过度激活,过度的心肌炎症则会造成心肌细胞凋亡及组织间质的纤维化,使得心室重构,并最终引发心力衰竭。因此调控心肌缺血再灌注损伤后炎症损伤,减轻心肌组织中炎症反应,是延缓心肌缺血再灌注损伤后心室重构、心功能恶化的关键因素之一[18]。上述国内外研究表明了间充质干细胞对于心肌缺血再灌注损伤炎症反应具有多重且不同的作用,在此基础上进一步拓展间充质干细胞的预处理、外泌体、基因修饰或联合药物运用。 2.2 间充质干细胞外泌体治疗心肌缺血再灌注损伤的相关研究 间充质干细胞外泌体是一类特殊的胞外囊泡,直径为30-150 nm,密度为1.09-1.18 g/mL,成分含有DNA、mRNA、miRNA、蛋白质、脂质等,因强大的信息传递潜力而备受关注,其可通过吞噬、胞膜融合、受体接触等方式,将内容物释放进其他细胞内参与调控,见图1[19]。"
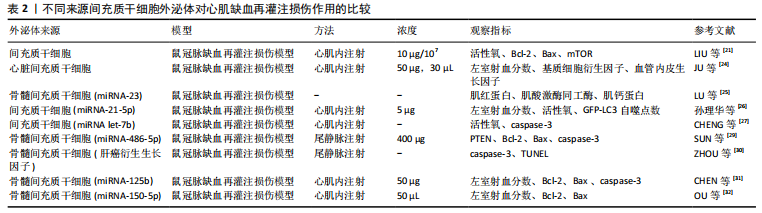
近年来诸多研究将间充质干细胞外泌体用于心肌梗死、心肌缺血再灌注等缺血性心脏疾病的治疗,证实了间充质干细胞外泌体具有心肌保护能力,且发现人源性间充质干细胞外泌体可缩小血栓,增强NADH、ATP水平,降低氧化应激水平,减轻炎症损伤并可增加Akt和GSK-3β的磷酸化(抗凋亡通路),降低c-JNK的磷酸化(促凋亡通路)[20]。LIU等[21]发现间充质干细胞通过外泌体降低大鼠心肌缺血再灌注损伤氧化应激反应,并减轻组织炎症损伤及减少心肌细胞凋亡,其机制可能与通过AMPK/mTOR和Akt/mTOR途径减轻心肌细胞自噬有关。然而,外泌体只是间充质干细胞胞外囊泡的一类,在间充质干细胞胞外囊泡研究中,ZHANG等[22]发现间充质干细胞胞外囊泡可促进内皮细胞成熟,调节心肌缺血再灌注损伤循环中巨噬细胞亚群,这一功能与间充质干细胞外泌体生物功能一致,二者最终均可改善心肌缺血再灌注损伤小鼠的心功能及心肌病理组织学变化。间充质干细胞胞外囊泡中包含成分复杂,GONG等[23]研究发现间充质干细胞胞外囊泡中基质衍生因子1可抑制心肌缺血再灌注损伤后心肌细胞自噬,并促进血管内皮细胞增殖及微血管再生。除外泌体外,间充质干细胞胞外囊泡还有更多方面值得深入研究。 不同源性间充质干细胞外泌体在心肌缺血再灌注损伤中的作用机制也大不相同,见表2。 在心肌组织修复及血管再生方面,JU等[24]研究发现心脏间充质干细胞外泌体是一种新颖的无细胞方式治疗心肌缺血再灌注损伤,心脏间充质干细胞可向心肌内传递外泌体促进新生血管生成及心肌细胞增殖,并修复损伤心脏组织,有效改善心功能。而LU等[25]研究发现抑制miRNA-23可诱导骨髓间充质干细胞向心肌细胞分化,从而达到修复心肌缺血再灌注损伤的效果。 在抑制炎症及减少心肌细胞凋亡等方面,孙理华等[26]研究发现大鼠心肌缺血再灌注损伤模型中,间充质干细胞外泌体miRNA-21-5p组的心肌细胞凋亡显著减少,左室短轴缩短率和左心室射血分数均显著提高,并指出其机制可能与通过调节心肌细胞自噬有关。CHENG等[27]研究发现间充质干细胞外泌体miRNA-let-7b可通过caspase-3通路调控心肌细胞凋亡及自噬,在心肌内注射miRNA-let-7b可保护移植物免受心肌缺血再灌注损伤的影响,增强心室功能,并促进心肌修复。 在间充质干细胞的研究发展史中,最初是从骨髓中提取并发现间充质干细胞的,因此骨髓间充质干细胞也被认为是间充质干细胞的最好来源,并将骨髓间充质干细胞作为衡量其他源性间充质干细胞的标准[28],而近年来骨髓间充质干细胞外泌体也引人关注。SUN等[29]研究发现骨髓间充质干细胞外泌体中miRNA-486-5p通过抑制PTEN基因表达,激活PI3K/AKT信号通路,达到抑制心肌细胞凋亡的作用。ZHOU等[30]研究发现骨髓间充质干细胞外泌体中肝癌衍生生长因子(HDGF)可通过激活蛋白激酶C减轻心肌缺血再灌注损伤,植入小鼠体内减小了心肌梗死面积及减少了心肌细胞凋亡,并改善心功能障碍。CHEN等[31]在大鼠心肌缺血再灌注损伤模型中,发现骨髓间充质干细胞外泌体中miRNA-125b可显著提高心肌细胞活力,降低心肌细胞凋亡率,并下调心肌组织中Bax和caspase-3凋亡因子表达,上调Bcl-2表达,并降低了白细胞介素1β、白细胞介素6及肿瘤坏死因子α等炎症因子水平,并指出去乙酰化转化酶(SIRT7)是miRNA-125b可能作用靶点,miRNA-125b显著下调心肌细胞中去乙酰化转化酶。OU等[32]研究发现骨髓间充质干细胞外泌体中miRNA-150-5p可抑制心肌重塑及心肌细胞凋亡,促进心肌组织修复。但目前骨髓间充质干细胞在取材、来源、伦理、供源等方面仍面临巨大挑战,哪种间充质干细胞更适合作为细胞组织工程的种子细胞及用于多种疾病的细胞治疗等问题,需要进一步更深入的研究。 近年来间充质干细胞外泌体研究是一大热点,间充质干细胞外泌体具有多种生物活性,其含有的mRNA、miRNA 以及各种抗凋亡、抑炎及促血管生成因子等,对心肌缺血再灌注损伤具有多种作用,包括心肌组织修复、促进血管再生、免疫调节及减轻炎症损伤等作用[33-35]。不同来源间充质干细胞外泌体对不同程度心肌细胞损伤的作用各不相同,但均可减轻心肌细胞损伤,其治疗效果并不亚于单纯使用间充质细胞移植治疗[36]。目前间充质干细胞外泌体还具备以下优势:①体外环境下外泌体可浓缩,因此可根据不同浓度要求输注间充质干细胞外泌体,且在间充质干细胞培养过程中可以反复提取外泌体;②目前对于间充质干细胞的基因修饰是一大热点,通过一些基因修饰过的间充质干细胞可塑造具有不同功能特点、不同作用机制的外泌体;③其较间充质干细胞更易于保存,使用更便捷;④可避免间充质干细胞移植治疗的缺陷,例如致肿瘤性、免疫原性以及人源性间充质干细胞伦理争议等缺点[37];⑤间充质干细胞外泌体可作为一种载体,运输各种不同类型的分子至靶组织,在药物尤其大分子递送方面具有良好的前景[38]。 2.3 间充质干细胞移植修复心肌缺血再灌注损伤后心肌组织修复与血管再生的变化 不同源性(脂肪、羊水、羊膜、骨髓、脐带等)间充质干细胞在心肌缺血再灌注损伤中可起到心肌组织修复及促进血管内皮细胞增殖、迁移的作用,改善心脏功能,进而促进血管管腔形成及血管再生,并减少心肌细胞及血管内皮细胞凋亡[39-42]。MONTANARI等[43]在大鼠异位心脏移植心肌缺血再灌注损伤模型中注射骨髓间充质干细胞,可早期改善心脏功能、改善心室重构,并减少心脏内膜纤维化,促进组织修复、增强血管新生及减少心肌细胞凋亡,有效延长了移植供体的存活时间。 随着间充质干细胞研究的不断深入,对间充质干细胞进行药物预处理、联合药物或化学物使用、基因修饰等研究逐步赶超单纯使用间充质干细胞,这些方法不仅增强了间充质干细胞的作用效力,更加强了间充质干细胞的归巢效应及安全性,为今后研究带来一种全新的思路。张红玉[44]研究发现用舒芬太尼预处理的大鼠脐血间充质干细胞,对比单纯干细胞移植能够更有效地减轻大鼠心肌梗死程度及心肌细胞凋亡,保护损伤的心肌。CIUFFREDA等[45]开发了一种酶可降解的肝素聚乙二醇水凝胶(H-HG),它能够增加间充质干细胞的滞留,并旁分泌可溶性因子,移植到心肌缺血再灌注损伤模型后心功能得到改善。LI等[46]在体外细胞研究中发现骨髓间充质干细胞条件培养基(BMSCs-CM)可以通过调节NOTChH/mTOR信号修复损伤的H9C2细胞(大鼠心肌细胞),并且延长其存活时间。禹丽等[47]研究发现硫氧还蛋白1基因修饰骨髓间充质干细胞移植组大鼠新生血管密度及心功能均显著恢复。王燕[48]研究发现兔心肌缺血再灌注损伤模型注射Akt基因修饰羊水间充质干细胞,可修复损伤心肌、减少梗死面积、改善心脏功能等,其修复作用机制可能与减少心肌细胞凋亡、促进血管新生、Akt基因转染增强细胞耐缺氧以及旁分泌作用等有关。间充质干细胞对体内环境的适应性及其对多种疾病的潜在治疗能力有待探究。"
| [1] 吴晓燕,苗琳,郑蕊,等.心肌缺血再灌注损伤的研究进展[J].中国临床药理学,2016,32(11):1043-1045,1056. [2] FRIEDENSTEIN AJ, PETRAKOVA KV, KUROLESOVA AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230-247. [3] 符武岛,曾敏,陈娟,等.表没食子儿茶素没食子酸酯通过抑制心肌细胞凋亡缓解心肌缺血再灌注损伤的机制研究[J].中国药房, 2019,30(16):2187-2192. [4] AHMED S, AHMED N, RUNGATSCHER A, et al. Cocoa Flavonoids Reduce Inflammation and Oxidative Stress in a Myocardial Ischemia-Reperfusion Experimental Model. Antioxidants (Basel). 2020;9(2):167. [5] 许毛,刘娜,苏燕胜.纤维蛋白原与心肌缺血再灌注损伤患者炎症反应的相关性分析[J].西安交通大学学报(医学版),2018,39(5): 698-702. [6] AL-SALAM S, HASHMI S. Myocardial Ischemia Reperfusion Injury: Apoptotic, Inflammatory and Oxidative Stress Role of Galectin-3. Cell Physiol Biochem. 2018;50(3):1123-1139. [7] SHIN EY, WANG L, ZEMSKOVA M, et al. Adenosine Production by Biomaterial-Supported Mesenchymal Stromal Cells Reduces the Innate Inflammatory Response in Myocardial Ischemia/Reperfusion Injury. J Am Heart Assoc. 2018;7(2):e006949. [8] HONG HS, KIM S, LEE S, et al. Substance-P Prevents Cardiac Ischemia-Reperfusion Injury by Modulating Stem Cell Mobilization and Causing Early Suppression of Injury-Mediated Inflammation. Cell Physiol Biochem. 2019;52(1):40-56. [9] ADINOLFI E, GIULIANI AL, DE MARCHI E, et al. The P2X7 receptor: A main player in inflammation. Biochem Pharmacol. 2018;151:234-244. [10] WANG J, CHEN Z, DAI Q, et al. Intravenously delivered mesenchymal stem cells prevent microvascular obstruction formation after myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2020;115(4):40. [11] KORKMAZ-ICÖZ S, LI S, HÜTTNER R, et al. Hypothermic perfusion of donor heart with a preservation solution supplemented by mesenchymal stem cells. J Heart Lung Transplant. 2019;38(3):315-326. [12] 张泽宇,苗迎春,张威,等.脂肪间充质干细胞移植对大鼠心肌缺血再灌注后炎症反应及心脏功能的影响[J].解放军医学院学报, 2016,37(4):381-385. [13] 陈士金,史钰芳,张博,等.脐带间充质干细胞移植对急性心肌缺血-再灌注损伤大鼠VEGF及MCP-1的影响[J].国际生物医学工程杂志,2017,40(6):453-456. [14] 梁贵友,余丽梅,巫宏坤,等.一种人羊膜间充质干细胞的应用:中国, CN201610235663.0[P].2016-07-20. [15] BOND TA, KARHUNEN V, WIELSCHER M, et al. Exploring the role of genetic confounding in the association between maternal and offspring body mass index: evidence from three birth cohorts. Int J Epidemiol. 2020;49(1):233-243. [16] ZHANG Z, TIAN H, YANG C, et al. Mesenchymal Stem Cells Promote the Resolution of Cardiac Inflammation After Ischemia Reperfusion Via Enhancing Efferocytosis of Neutrophils. J Am Heart Assoc. 2020; 9(5):e014397. [17] WEI Z, QIAO S, ZHAO J, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019; 232:116632. [18] PRABHU SD, FRANGOGIANNIS NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119(1):91-112. [19] ZHENG G, HUANG R, QIU G, et al. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374(1):1-15. [20] 何尤夫,石蓓.干细胞源外泌体治疗缺血性心脏病的近况[J].临床与病理杂志,2017,37(2):422-428. [21] LIU L, JIN X, HU CF, et al. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell Physiol Biochem. 2017;43(1):52-68. [22] ZHANG N, SONG Y, HUANG Z, et al. Monocyte mimics improve mesenchymal stem cell-derived extracellular vesicle homing in a mouse MI/RI model. Biomaterials. 2020;255:120168. [23] GONG XH, LIU H, WANG SJ, et al. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J Cell Physiol. 2019;234(8): 13878-13893. [24] JU C, SHEN Y, MA G, et al. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes Promotes Repair in Ischemic Myocardium. J Cardiovasc Transl Res. 2018;11(5):420-428. [25] LU M, XU Y, WANG M, et al. MicroRNA-23 inhibition protects the ischemia/reperfusion injury via inducing the differentiation of bone marrow mesenchymal stem cells into cardiomyocytes. Int J Clin Exp Pathol. 2019;12(3):1060-1069. [26] 孙理华,王娟,张岳,等.间充质干细胞来源的外泌体通过microRNA-21-5p调节心脏自噬并影响心肌缺血大鼠的心脏功能[J].中国比较医学杂志,2020,30(5):88-96. [27] CHENG J, ZHANG P, JIANG H. Let-7b-mediated pro-survival of transplanted mesenchymal stem cells for cardiac regeneration. Stem Cell Res Ther. 2015;6:216. [28] ULLAH I, SUBBARAO RB, RHO GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2):e00191. [29] SUN XH, WANG X, ZHANG Y, et al. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23-32. [30] ZHOU Y, CHEN P, LIU Q, et al. Hepatoma-Derived Growth Factor Secreted from Mesenchymal Stem Cells Reduces Myocardial Ischemia-Reperfusion Injury. Stem Cells Int. 2017;2017:1096980. [31] CHEN Q, LIU Y, DING X, et al. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. 2020;465(1-2):103-114. [32] OU H, TENG H, QIN Y, et al. Extracellular vesicles derived from microRNA-150-5p-overexpressing mesenchymal stem cells protect rat hearts against ischemia/reperfusion. Aging (Albany NY). 2020; 12(13):12669-12683. [33] ZHANG B, WU X, ZHANG X, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4(5):513-522. [34] MA J, ZHAO Y, SUN L, et al. Exosomes Derived from Akt-Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor D. Stem Cells Transl Med. 2017;6(1):51-59. [35] ROŞCA AM, ŢUŢUIANU R, TITORENCU ID. Mesenchymal stromal cells derived exosomes as tools for chronic wound healing therapy. Rom J Morphol Embryol. 2018;59(3):655-662. [36] 刘卒,韩燊,李亚雄,等.不同干细胞来源外泌体在心血管疾病治疗中的应用、作用及问题[J].中国组织工程研究,2020,24(19): 3063-3070. [37] SUN L, XU R, SUN X, et al. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18(3):413-422. [38] PITT JM, KROEMER G, ZITVOGEL L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126(4):1139-1143. [39] GNECCHI M, DANIELI P, MALPASSO G, et al. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016; 1416:123-146. [40] XU H, DONG H, ZHAO M. Effects of human umbilical cord mesenchymal stem cells on vascular endothelial growth factor and IL-6 expression in tissue of AMI rats. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017; 29(6):511-514. [41] GU W, HONG X, POTTER C, et al. Mesenchymal stem cells and vascular regeneration. Microcirculation. 2017;24(1):e12324. [42] BARILE L, MOCCETTI T, MARBÁN E, et al. Roles of exosomes in cardioprotection. Eur Heart J. 2017;38(18):1372-1379. [43] MONTANARI S, DAYAN V, YANNARELLI G, et al. Mesenchymal stromal cells improve cardiac function and left ventricular remodeling in a heart transplantation model. J Heart Lung Transplant. 2015;34(11): 1481-1488. [44] 张红玉.舒芬太尼联合脐血间充质干细胞移植对梗死心肌的保护作用[J].中国组织工程研究,2016,20(41):6164-6170. [45] CIUFFREDA MC, MALPASSO G, CHOKOZA C, et al. Synthetic extracellular matrix mimic hydrogel improves efficacy of mesenchymal stromal cell therapy for ischemic cardiomyopathy. Acta Biomater. 2018;70:71-83. [46] LI X, XIE X, YU Z, et al. Bone marrow mesenchymal stem cells-derived conditioned medium protects cardiomyocytes from hypoxia/reoxygenation-induced injury through Notch2/mTOR/autophagy signaling. J Cell Physiol. 2019;234(10):18906-18916. [47] 禹丽,许兰莹,黄启星,等.硫氧还蛋白1基因修饰骨髓间充质干细胞对心肌梗死大鼠血管生成及心功能恢复的研究[J].临床和实验医学杂,2019,18(17):1808-1811. [48] 王燕.Akt基因修饰羊水间充质干细胞移植对兔心肌缺血再灌注损伤的作用研究[D].上海:上海交通大学,2016. |
| [1] | Xiang Xinjian, Liu Fang, Wu Liangliang, Jia Daping, Tao Yue, Zhao Zhengnan, Zhao Yu. High-dose vitamin C promotes the survival of autologous fat transplantation in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1298-1302. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1316-1322. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1323-1329. |
| [4] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1330-1335. |
| [5] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1183-1190. |
| [6] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1191-1197. |
| [7] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1068-1074. |
| [8] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1133-1140. |
| [9] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1141-1150. |
| [10] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1151-1155. |
| [11] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1156-1162. |
| [12] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1163-1169. |
| [13] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1170-1176. |
| [14] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1177-1182. |
| [15] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1086-1092. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||