Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (20): 3188-3194.doi: 10.3969/j.issn.2095-4344.1156
Previous Articles Next Articles
Fracture healing mechanism at molecular signaling pathway and cellular levels
Yang Nan1, Chen Yueping2, Zhang Xiaoyun2
- 1Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China; 2Department of Traumatic Orthopedics and Hand Surgery, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China
-
Online:2019-07-18Published:2019-07-18 -
Contact:Chen Yueping, Doctoral supervisor, Chief physician, Department of Traumatic Orthopedics and Hand Surgery, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China Zhang Xiaoyun, Master, Attending physician, Department of Traumatic Orthopedics and Hand Surgery, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
About author:Yang Nan, Master candidate, Guangxi University of Chinese Medicine, Nanning 530001, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81760796 (to CYP)| the Natural Science Foundation of Guangxi Zhuang Autonomous Region, No. 2015GXNSFAA139136 (to CYP)| the Basic Ability Improvement Project of Young Teachers in Guangxi Universities, No. KY2016YB204 (to ZXY)| the Key Project of Health Department of Guangxi Zhuang Autonomous Region, No. S201419-05 (to CYP)
CLC Number:
Cite this article
Yang Nan, Chen Yueping, Zhang Xiaoyun. Fracture healing mechanism at molecular signaling pathway and cellular levels[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(20): 3188-3194.
share this article
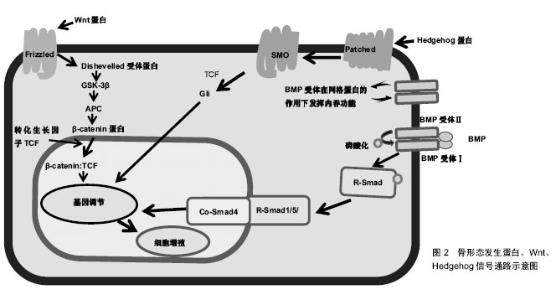
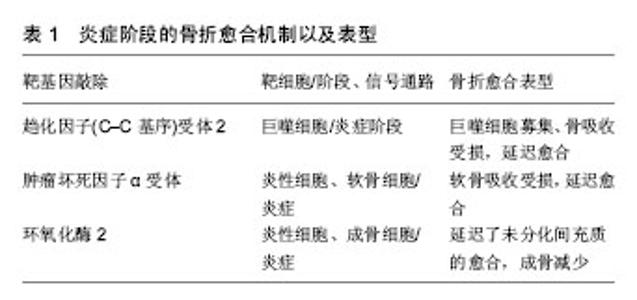
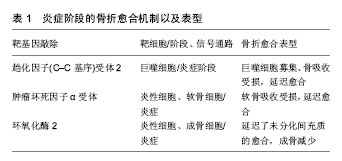
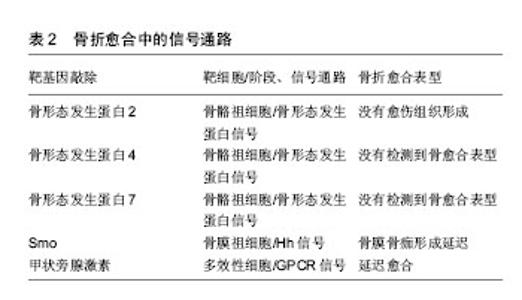
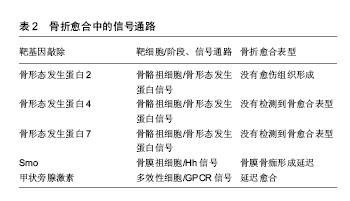
在炎症阶段中,涉及先天免疫的部分细胞类型对骨折愈合有积极作用,如通过灭活实验小鼠趋化因子(C-C基序)受体2基因,导致趋化因子受体2失活,使招募至骨折部位的巨噬细胞减少,与野生小鼠相比,骨痂内的破骨细胞数量并没有减少,但是其骨吸收能力下降,骨重塑明显延迟[7];而部分成分可能具有负面影响,如Wang[8]及其同事发现Toll样受体4基因失活加速了颅骨缺损的愈合,进一步揭示了骨骼和免疫系统之间复杂的相互作用。适应性免疫系统中不同细胞对骨修复影响也不尽相同,小鼠截骨模型中CD8T细胞的耗竭可使骨折再生增强[4],而γδT细胞对骨折愈合有积极作用[9]。 肿瘤坏死因子α也是在骨折愈合过程中起关键作用的炎症递质。肿瘤坏死因子α是一个可由多种细胞表达并起积极作用的因子,其中包括炎症细胞、骨骼谱系细胞[6],所以需要发展特殊靶向研究方法帮助更好地确认其对不同细胞类型、修复阶段的作用和在不同浓度下的影响。在骨再生的早期阶段,肿瘤坏死因子α促进干细胞的募 集[10];在修复的后期阶段,肿瘤坏死因子α调节骨痂内的软骨吸收,导致缺乏肿瘤坏死因子α受体的小鼠愈合受 损[11]。肿瘤坏死因子α在低浓度下促进成骨分化,在高浓度下具有抑制作用[10]。尽管炎症反应对于启动修复联锁反应必不可少,但是过度或延长的炎症反应对于修复是有害的,如多创伤患者观察到的并发症所示[2,12]。临床中同样观察到糖尿病患者骨折不易愈合,经过研究发现糖尿病患者的机体会长期处于慢性炎症状态,肿瘤坏死因子α水平持续升高,导致软骨细胞和间充质干细胞在早期凋亡。而且高葡萄糖和晚期糖基化终产物增强了肿瘤坏死因子α在体外的作用,破坏微血管内皮细胞增殖和血管形成,并刺激细胞凋亡。进一步研究其机制发现转录因子FoxO1调控了肿瘤坏死因子、高葡萄糖和晚期糖基化终产物的作用过程,提高了凋亡相关因子p21和caspase-3(细胞凋亡过程中最主要的终末剪切酶)的表达[13]。这些研究表明了过度的炎症和患有其他基础疾病对骨折愈合的负面影响,将会为临床制定个体化治疗方案提供依据。 通过对一些缺乏特异性炎症因子的细胞系研究,也有助于阐明骨折修复过程中炎症递质对软骨和骨的影响。如前列腺素是其中关键的非炎症分子,通过促进血管生成和刺激破骨细胞、成骨细胞来促进骨再生[14]。前列腺素合成由环氧化酶催化,而环氧化酶2在骨折后随即被刺激表达,导致骨折后前列腺素合成增多[14-15]。对实验小鼠的分析表明,还发现环氧化酶2也可以通过诱导间充质干细胞的核心结合因子a1(cbfa1)和osterix(OSX)蛋白参与成骨过程促进骨愈合。环氧化酶2(-/-)小鼠成骨细胞明显减少,软骨板上没有初级骨形成,骨折愈合也明显延迟。但前列腺素激动剂(前列腺素E2)的加入弥补这一缺陷,骨形态发生蛋白2也可提高了环氧化酶2(-/-)和野生型小鼠体内cbfa1和OSX含量[15-16],由此说明骨折修复的过程的复杂性。 在骨折后临床常规用药方案中,需要注意非类固醇抗炎药的使用,这是用于治疗骨折疼痛的药物,但又是环氧化酶2的有效抑制剂,可以通过抑制环氧化酶2阻碍骨折愈合[16]。 2.2 信号通路 2.2.1 骨形态发生蛋白信号通路 骨形态发生蛋白是属于转化生长因子β家族的分泌信号蛋白,存在于多种细胞中,不仅在骨骼发育和生理中起着重要的作用,也参与了许多器官的发育,已经在实验及临床阶段证明可以在骨折的不同愈合阶段和修复不同细胞上发挥作用。骨形态发生蛋白被前蛋白肽酶催化后产生成熟的二聚体,然后又结合2个拷贝的Ⅰ型和Ⅱ型骨形态发生蛋白受体,产生异六聚体复合物。骨形态发生蛋白同源物与其同源受体的结合导致Ⅰ型受体在Ⅱ型受体的作用下磷酸化。然后激活磷酸化后的骨形态发生蛋白受体和Smad1/5/8蛋白的结合体,这些蛋白与Smad4组成二聚体并积聚在细胞核中,它们一起介导骨形态发生蛋白调节基因表达的变化[17],见图2及表2。骨形态发生蛋白可以诱导成骨,已被纯化应用于临床,然而治疗成本高,又有副反应,如导致异位骨形成、骨溶解等[17]。虽然通过前人研究发现肉毒杆菌毒素A可以诱导短期肌肉萎缩导致成骨受损,皮质间缺损可以诱导骨膜成骨,肌肉内注射骨形态发生蛋白可以刺激异位骨形成。Ausk[12]及其同事研究证明了肉毒杆菌毒素A可以通过抑制神经肌肉,减轻与皮质间缺损、骨形态发生蛋白2病理性诱导相关的异位骨化情况,找到解决异位骨化问题的新思路。但为了规范临床对骨形态发生蛋白的使用标准,现在研究通过(条件)基因敲除法发现骨形态发生蛋白家族不同亚型的作用。"
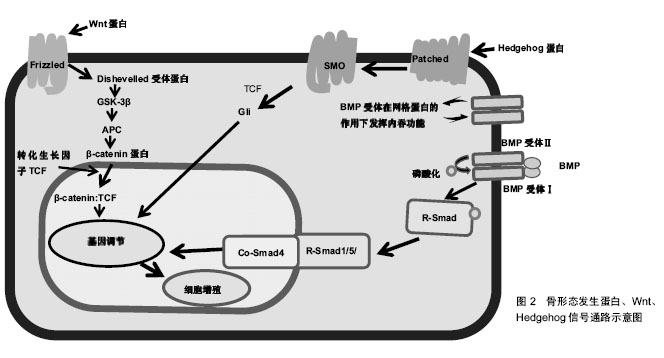

骨形态发生蛋白作用广泛,经过众多实验和临床研究发现,大多数骨形态发生蛋白和骨形态发生蛋白受体基因敲除的小鼠在早期胚胎阶段会死亡的,或者表现出骨骼的异常状态[18]。骨形态发生蛋白7基因敲除小鼠表现出围产期致死性,而骨形态发生蛋白6基因敲除小鼠可存活[19]。Tsuji等[20-22]使用了条件基因敲除方法,通过将携带这些骨形态发生蛋白基因的固定等位基因的小鼠与Prx1-Cre小鼠杂交,仅使肢体中的骨形态发生蛋白2、骨形态发生蛋白4、骨形态发生蛋白7基因失活。对骨形态发生蛋白2、骨形态发生蛋白7、骨形态发生蛋白4基因使用条件敲除后的小鼠表现出正常的肢体骨,表明其在长骨发育中多余。但是骨形态发生蛋白4和骨形态发生蛋白7 条件基因敲除小鼠骨折后可自行愈合,但骨形态发生蛋白2条件基因敲除小鼠会有自发性骨折并不愈合,表明骨形态发生蛋白2在骨修复中起重要作用[20]。现在研究发现骨形态发生蛋白2基因在染色体20p 12区,属于编码转化生长因子β信号蛋白的基因超家族。根据临床观察研究,Tan[23]及其同事报告了12例骨形态发生蛋白2基因突变个体,12例病例均显示出一致的以身材矮小、骨骼和心脏异常为特征的表型,没有神经缺陷的独特表现,而且进一步实验研究显示骨形态发生蛋白2通过激活骨膜内的祖细胞在骨修复的早期阶段发挥关键作用。 2.2.2 Wnt信号通路 Wnt蛋白是一类分泌蛋白,对早期胚胎发生、器官发生和组织修复(包括骨骼)至关重要,也与骨形态发生蛋白信号通路相互作用[24]。Wnt蛋白结合其膜受体Fzd1、Fzd2、Fzd4和Fzd5,以及它们的共同受体,即低密度脂蛋白受体相关蛋白5和低密度脂蛋白受体相关蛋白6[25],见图2,这种结合可通过β-钙通道蛋白的核转位激活经典的信号传导通路。分泌蛋白1和硬化素(Sclerostin)通过与低密度脂蛋白受体相关蛋白5/6受体结合而起到Wnt信号拮抗剂的作用[24,26]。因为Wnt信号通路的改变可影响骨折愈合,通过抑制Wnt信号的拮抗剂(如抗分泌蛋白1的抗体)来提高Wnt信号通路作用,可以促进骨折修复[24,26-28],所以Wnt信号通路,是潜在的可供选择的骨合成代谢途径。低密度脂蛋白受体相关蛋白5功能紊乱将会通过影响Wnt通路来影响骨形成,如在人类高骨量疾病中,发现是低密度脂蛋白受体相关蛋白5基因错意突变导致[26]。而骨质疏松假神经胶质瘤综合征这种以低骨量和失明为特征的常染色体隐性疾病则是由低密度脂蛋白受体相关蛋白5的基因丧失突变引起[25]。 运用β-Catenin基因条件敲除和敲入方法,研究Wnt/β-Catenin信号在骨折愈合的不同阶段表达出的不同功能[26]。如在骨折愈合的早期阶段,β-Catenin可以驱动间充质细胞分化为成骨细胞和软骨细胞系;在分化后期的成骨细胞系中,β-Catenin信号转导减少,成骨细胞成熟为骨细胞[27]。因此,当在骨折修复的初始阶段之后给予锂(β-Catenin的激活剂)治疗增强骨折修复[28]。 2.2.3 Hedgehog信号通路 Indian hedgehog(Ihh)也是骨折愈合过程中主调控软骨细胞和成骨细胞分化的因子。hedgehog(Hh)信号通路是通过将Hh蛋白结合到其受体跨膜蛋白patched(Ptch)上来触发的,从而解除Ptch对跨膜蛋白smoothened(Smo)的抑制,导致Smo激活[29],见图2及表2。Hh信号通路在骨折修复过程中在成骨细胞中起着关键作用,抑制成骨细胞中的信号通路可以导致骨折部位基质减少,而激活可以增加基质沉积[30]。Ihh表达水平在骨折愈合的软骨形成期增加[30]。Kazmers[31]及其同事研究发现通过抑制Hh信号发现,Hh信号通过调节骨生成和血管生成在骨折愈合过程中直接发挥作用。 2.2.4 甲状旁腺激素信号 在骨愈合过程中涉及的其他信号通路中,甲状旁腺激素信号通过增加成骨细胞的数量来刺激骨形成,见表2。甲状旁腺激素和甲状旁腺激素相关肽的作用是由G蛋白偶联受体介导的[32]。Ren[33]和同事们发现,甲状旁腺激素-null小鼠内源性甲状旁腺激素的缺乏导致软骨和骨痂形成减少,同时还会下调成骨相关基因表达,以及减少软骨内成骨、成骨细胞骨形成和破骨细胞骨吸收。这些结果表明内源性甲状旁腺激素在骨折愈合中起着重要作用[33]。Esen[34]及其同事发现甲状旁腺激素通过刺激胰岛素样生长因子1信号抑制三羧酸循环、促进有氧糖酵解、刺激氧化有助于血管形成、骨合成代谢。而且骨吸收时,从骨基质中释放的胰岛素样生长因子1在骨重塑过程中还可以刺激成骨细胞分化。甲状旁腺激素是唯一一种美国食品和药物管理局批准的用于骨质疏松症患者来增加骨形成的分子。研究发现间断性甲状旁腺激素治疗可抑制成骨细胞凋亡,这可能与间断性甲状旁腺激素治疗后成骨细胞数量增加和随后骨形成增加有关[32,34-35]。但是通过甲状旁腺功能亢进症可能出现骨折延迟或不愈合临床现状,开展相关研究发现是甲状旁腺激素影响了软骨内骨化所致。如持续注射甲状旁腺激素的小鼠出现骨折延迟愈合,但最终结果显示加强了骨骼生物力学性能[36]。 Wnt和甲状旁腺激素可以通过互补途径刺激骨形成。间断性甲状旁腺激素正向调节骨骼微环境中的局部Wnt信号,增加对骨形成、重塑的刺激[35]。而硬化素是一种强有力的Wnt信号拮抗剂,这是一种几乎只由成骨细胞和骨细胞表达的蛋白[37]。间断性甲状旁腺激素不仅可以调节硬化素、分泌蛋白1的表达,还调节其他分泌信号的拮抗剂的表达。这些拮抗剂的减少也可能介导了间断性甲状旁腺激素诱导骨形成的过程[38]。这些结果说明了骨修复过程中多种信号通路之间相互作用。 2.3 细胞 Harold Frost在50年前将基本多细胞单位定义为在骨重塑过程和骨自我更新中起关键作用并相互影响的细胞群,其中主要是破骨细胞和成骨细胞之间、成骨细胞和骨细胞之间以及成骨细胞之间相互影响。随着研究的深入,发现在骨重塑过程中发挥重要作用的不止包括骨特有的细胞,而且基本多细胞单位也不局限于影响骨代谢[39]。鉴于成骨细胞和破骨细胞之间特殊联系,此小结综述成骨细胞对破骨细胞分化的影响以及泛素依赖性蛋白水解系统对成骨细胞的影响。 2.3.1 破骨细胞 破骨细胞来源于造血干细胞,属于单核细胞/巨噬细胞谱系。破骨细胞在骨折中的作用开始于破骨细胞前体细胞(Osteoclast precursors)在骨折修复炎症阶段从血液循环中被招募,并在核因子κB受体活化因子配体(NF-κB ligand,RANKL)的刺激下留在损伤的骨表面,之后在骨表面迁移至骨衬细胞层、成骨细胞层下的过程中由分化因子诱导分化、增殖为抗酒石酸酸性磷酸酶阳性单核细胞,最终分化融合成多核破骨细胞,发挥骨吸收作用,骨吸收完成后细胞凋亡[40]。 在整个过程中成骨细胞分泌骨保护素(抑制分化)、巨噬细胞集落刺激因子和RANKL参与调控破骨细胞分化,巨噬细胞集落刺激因子、RANKL(分化因子)直接参与诱导分化[41]。最后成骨细胞通过ephrinB2-EphB4信号也参与调控破骨细胞的凋亡[42]。 2.3.2 成骨细胞 骨髓基质中的间充质基质细胞、骨髓窦中的周细胞通过表达转录因子Runx2和OSX达实现对成骨细胞的控制和分化,随着碱性磷酸酶、1型胶原和非胶原蛋白的表达,骨基质沉积、矿化[43-44]。在骨形成期结束时,一些成骨细胞或凋亡或成为流动的骨衬细胞,而另一些作为调控骨重塑的骨细胞嵌入骨基质中[44]。 在组织水平上骨形成的活性取决于分化的成骨细胞的数量和功能。而任何相关细胞和分子的代谢性、遗传性或致癌性疾病都能影响成骨细胞的募集、功能或存活,如更年期性激素的缺乏、衰老、糖尿病、糖皮质激素治疗、多发性骨髓瘤等等。既然已经确定了这些致病因素,目前的目标是找到特殊靶向方法,研发有效的治疗策略来促进骨骼老化或骨折延迟愈合时期的骨形成。在此背景下,考虑促进成骨细胞发生的若干因素和信号通路,这包括胰岛素样生长因子1、骨形态发生蛋白、成纤维细胞生长因子、转化生长因子β、Notch和Wnt信号[17,24,34,45]。 另一种增加成骨细胞生成的新方法是针对泛素依赖性蛋白水解系统,其中E3泛素连接酶是参与泛素化的种类最丰富的酶组。E3泛素连接酶Smurf1主要介导成骨细胞转录因子Runx2,信号蛋白Jub2、磷酸化的促分裂原活化蛋白激酶2以及可被骨形态发生蛋白2激活的Smad1和Smad4的泛素化和降解。Wwp1和Schnurri-3介导了Runx2的降解,导致成骨细胞分化和骨形成减少。合成代谢因子骨形态发生蛋白2刺激Runx2乙酰化,从而防止Smurf1诱导的Runx2降解。分解代谢因子肿瘤坏死因子a增强了Smurf1的表达,导致Runx2降解。持续性甲状旁腺激素增加Smurf1的表达,而间断性甲状旁腺激素可防止b-TcCP1降解活性转录因子4,从而促进骨形成。在骨骼中,泛素依赖性蛋白水解系统控制关键调节成骨细胞的因子如RTKs、骨形态发生蛋白2、Runx2、β-Catenin和GLI2(调节骨形态发生蛋白2表达的转录因子)的降解,可能成为促进成骨和减少肿瘤发生的潜在治疗策略[46]。"

| [1] Einhorn TA,Gerstenfeld LC.Fracture healing:mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45-54.[2] Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133-143.[3] Shiu HT, Leung PC, Ko CH. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J Tissue Eng Regen Med. 2018;12(4):e1911-e1925.[4] Reinke S, Geissler S, Taylor WR, et al. Terminally differentiated CD8?T cells negatively affect bone regeneration in humans. Sci Transl Med. 2013;5(177):177ra36.[5] Dai Y, Li X, Wu R,et al. Macrophages of different phenotypes influence the migration of bmscs in plga scaffolds with different pore size. Biotechnol J. 2018;13(1). doi: 10.1002/biot.201700297.[6] Chan JK, Glass GE, Ersek A, et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol Med. 2015;7(5):547-561.[7] Xing Z,Lu C,Hu D,et al. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3(7-8):451-458.[8] Wang D,Gilbert JR,Cray JJ,et al.Accelerated calvarial healing in mice lacking Toll-like receptor 4.PLoS ONE. 2012;7(10): e46945.[9] Kalyan S. It may seem inflammatory,but some T cells are innately healing to the bone. J Bone Miner Res. 2016;31(11): 1997-2000.[10] Glass GE,Chan JK,Freidin A,et al.TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci USA. 2011;108(4):1585-1590.[11] Gerstenfeld LC,Cho TJ,Kon T,et al.Impaired fracture healing in the absence of TNF-alpha signaling:the role of TNF-alpha in endochondral cartilage resorption.J Bone Miner Res. 2003;18(9):1584-1592.[12] Ausk BJ,Gross TS,Bain SD. Botulinum Toxin-induced Muscle Paralysis Inhibits Heterotopic Bone Formation.Clin Orthop Relat Res. 2015;473(9):2825-2830.[13] Lim JC,Ko KI,Mattos M,et al.TNFα contributes to diabetes impaired angiogenesis in fracture healing.Bone. 2017;99: 26-38.[14] Lu LY,Loi F,Nathan K,et al.Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway.J Orthop Res. 2017;35(11): 2378-2385.[15] Zhang X,Schwarz EM,Young DA,et al.Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair.J Clin Invest. 2002;109(11):1405-1415.[16] Wang JH,Liu YZ,Yin LJ,et al.BMP9 and COX-2 form an important regulatory loop in BMP9-induced osteogenic differentiation of mesenchymal stem cells.Bone. 2013;57(1): 311-321.[17] Brazil DP,Church RH,Surae S,et al.BMP signalling:agony and antagony in the family.Trends Cell Biol.2015;25(5):249-264.[18] Oryan A,Alidadi S,Moshiri A,et al.Bone morphogenetic proteins:a powerful osteoinductive compound with non-negligible side effects and limitations.Biofactors. 2014; 40(5):459-481. [19] Solloway MJ,Dudley AT,Bikoff EK,et al. Mice lacking Bmp6 function. Dev Genet.1998;22(4):321-339.[20] Tsuji K,Bandyopadhyay A,Harfe BD,et al.BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing.Nat Genet. 2006;38(12): 1424-1429.[21] Tsuji K,Cox K,Bandyopadhyay A,et al.BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am. 2008;90 Suppl 1:14-18.[22] Tsuji K,Cox K,Gamer L,et al.Conditional deletion of BMP7 from the limb skeleton does not affect bone formation or fracture repair.J Orthopa Res.2010;28(3):384-389.[23] Tan TY, Gonzaga-Jauregui C, Bhoj EJ, et al. Monoallelic bmp2 variants predicted to result in haploinsufficiency cause craniofacial,skeletal,and cardiac features overlapping those of 20p12 deletions. Am J Hum Genet.2017;101(6):985-994.[24] Ellies DL,Viviano B,Mccarthy J,et al.Bone density ligand, Sclerostin,directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2010;21(11): 1738-1749.[25] Gong Y,Slee RB,Fukai N,et al.LDL receptor-related protein 5(LRP5)affects bone accrual and eye development.Cell. 2001;107(4):513-523.[26] Niziolek PJ, MacDonald BT, Kedlaya R, et al. High bone mass-causing mutant lrp5 receptors are resistant to endogenous inhibitors in vivo. J Bone Miner Res. 2015; 30(10):1822-1830.[27] Yan C,Whetstone HC,Lin AC,et al. Beta-catenin signaling plays a disparate role in different phases of fracture repair:implications for therapy to improve bone healing. Plos Med. 2007;4(7):e249.[28] Jin H,Wang B,Li J,et al.Anti-DKK1 antibody promotes bone fracture healing through activation of β-catenin signaling. Bone. 2015;71:63-75.[29] Long F,Chung UI,Ohba S,et al.Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton.Development. 2004;131(6):1309-1318.[30] Baht GS,Silkstone D,Nadesan P,et al.Activation of hedgehog signaling during fracture repair enhances osteoblastic- dependent matrix formation.J Orthop Res. 2014;32(4): 581-586.[31] Kazmers NH,McKenzie JA,Shen TS,et al.Hedgehog signaling mediates woven bone formation and vascularization during stress fracture healing.Bone. 2015;81:524-532.[32] Esbrit P, Alcaraz MJ. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol.2013;85(10): 1417-1423.[33] Ren Y,Bo L,Feng Y,et al.Endogenous PTH Deficiency Impairs Fracture Healing and Impedes the Fracture-Healing Efficacy of Exogenous PTH(1-34).Plos One.2011;6(6):e23060.[34] Esen E,Lee SY,Wice BM,et al.PTH Promotes Bone Anabolism by Stimulating Aerobic Glycolysis via IGF Signaling.J Bone Miner Res. 2015;30(11):1959-1968.[35] Grosso MJ,Courtland HW,Yang X,et al.Intermittent PTH administration and mechanical loading are anabolic for periprosthetic cancellous bone.J Orthop Res. 2015;33(2): 163-173.[36] Yukata K,Kanchiku T,Egawa H,et al.Continuous infusion of PTH delayed fracture healing in mice. Sci Rep. 2018;8(1): 13175.[37] Bodine PV, Seestaller-Wehr L, Kharode YP, et al. Bone anabolic effects of parathyroid hormone are blunted by deletion of the Wnt antagonist secreted frizzled-related protein-1. J Cell Physiol. 2007;210(2):352-357.[38] Guo J,Liu M,Yang D,et al.Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation.Cell Metab. 2010;11(2):161-171.[39] Sims NA, Civitelli R. Cell-cell signaling: broadening our view of the basic multicellular unit.Calcif Tissue Int. 2014;94: 2-3.[40] Kenichi N. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010;67(23):4001-4009.[41] Ren H, Ren H, Li X, et al. Effects of intermedin on proliferation, apoptosis and the expression of OPG/RANKL/M-CSF in the MC3T3-E1 osteoblast cell line. Mol Med Rep. 2015;12(5): 113-117.[42] 王丽美. ephrinB2-EphB4正向信号介导的TNF-α调控成骨细胞分化过程中的作用研究[D]. 济南:山东大学, 2017.[43] Forlino A,Marini JC.Osteogenes is imperfecta.Lancet. 2016; 387(10028):1657-1671.[44] Marie PJ.Osteoblast dysfunctions in bone diseases:from cellular and molecular mechanisms to therapeutic strategies. Cell Mol Life Sci. 2015;72(7):1347-1361.[45] 官建中,刘亚军,王照东,等. 联合应用外源性TGF-β2及IGF-1对大鼠骨折愈合的影响[J]. 中国矫形外科杂志, 2016, 24(2): 165-170.[46] Sévère N, Dieudonné FX. E3 ubiquitin ligase-mediated regulation of bone formation and tumorigenesis.Cell Death Dis. 2013;4:e463. |
| [1] | Yang Weiqiang, Ding Tong, Yang Weike, Jiang Zhengang. Combined variable stress plate internal fixation affects changes of bone histiocyte function and bone mineral density at the fractured end of goat femur [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 890-894. |
| [2] | Li Shibin, Lai Yu, Zhou Yi, Liao Jianzhao, Zhang Xiaoyun, Zhang Xuan. Pathogenesis of hormonal osteonecrosis of the femoral head and the target effect of related signaling pathways [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 935-941. |
| [3] | Cheng Shigao, , Wang Wanchun, Jiang Dong, Li Tengfei, Li Xun, Ren Lian. Comparison of the standard and long-stem bone cement prosthesis replacement in the treatment of intertrochanteric fractures in elderly patients [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 362-367. |
| [4] | Wang Qiufei, Gu Ye, Peng Yuqin, Xue Feng, Ju Rong, Zhu Feng, Wang Yijun, Geng Dechun, Xu Yaozeng. Effect of Wnt/beta-catenin signaling pathway on osteoblasts under the action of wear particles [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3894-3901. |
| [5] | Liu Jinfu, Zeng Ping, Nong Jiao, Fan Siqi, Feng Chengqin, Huang Jiaxing. Integrative analysis of biomarkers and therapeutic targets in synovium of patients with osteoarthritis by multiple microarrays [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3690-3696. |
| [6] | Yang Caihui, Liu Qicheng, Dong Ming, Wang Lina, Zuo Meina, Lu Ying, Niu Weidong. Serine/threonine protein kinases can promote bone destruction in mouse models of chronic periapical periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3654-3659. |
| [7] | Fan Junchao, Chen Yong, Song Junjie. Sevoflurance combined with xenon pretreatment protects against spinal cord ischemia-reperfusion injury in a rat model [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3660-3665. |
| [8] | Huo Hua, Cheng Yuting, Zhou Qian, Qi Yuhan, Wu Chao, Shi Qianhui, Yang Tongjing, Liao Jian, Hong Wei. Effects of drug coating on implant surface on the osseointegration [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3558-3564. |
| [9] | Jiang Shengyuan, Li Dan, Jiang Jianhao, Shang-you Yang, Yang Shuye. Biological response of Co2+ to preosteoblasts during aseptic loosening of the prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(21): 3292-3299. |
| [10] | Zhong Yuanming, He Bingkun, Wu Zhuotan, Wu Sixian, Wan Tong, Zhong Xifeng. An exploration on the mechanism of Shaoyao Gancao Decoction in treating early pain of lumbar disc herniation based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(20): 3194-3201. |
| [11] | Chen Xinling, Wang Shenglan. Cell autophagy, pathway, regulation and its multiple correlations with pulmonary hypertension [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 311-316. |
| [12] | Wei Qin, Zhang Xue, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Jia Qiyu, Ma Chuang. Platelet-derived growth factor-BB induces the differentiation of rat bone marrow mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2953-2957. |
| [13] | Guo Zhibin, Wu Chunfang, Liu Zihong, Zhang Yuying, Chi Bojing, Wang Bao, Ma Chao, Zhang Guobin, Tian Faming. Simvastatin stimulates osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2963-2968. |
| [14] | Yan Xiurui, Tao Jin, Liang Xueyun. Mechanism by which exosomes from human fetal placental mesenchymal stem cells protect lung epithelial cells against oxidative stress injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2994-2999. |
| [15] | Su Mingzhu, Ma Yuewen. Radial extracorporeal shock wave therapy regulates the proliferation and differentiation of neural stem cells in the hippocampus via Notch1/Hes1 pathway after cerebral ischemia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3009-3015. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||