Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (8): 1301-1306.doi: 10.3969/j.issn.2095-4344.2017.08.026
Previous Articles Next Articles
Generation and application of three-dimensional cell traction force
Liu Chen, Chen Wei-yi, Li Xiao-na, Wang Xiao-jun
- College of Mechanics, Taiyuan University of Technology, Taiyuan 030024, Shanxi Province, China
-
Received:2016-12-13Online:2017-03-18Published:2017-04-14 -
Contact:Chen Wei-yi, M.D., Professor, Doctoral supervisor, College of Mechanics, Taiyuan University of Technology, Taiyuan 030024, Shanxi Province, China -
About author:Liu Chen, Master, College of Mechanics, Taiyuan University of Technology, Taiyuan 030024, Shanxi Province, China -
Supported by:the National Natural Science Foundation of China, No. 31271005, 11402161 and 11402162
CLC Number:
Cite this article
Liu Chen, Chen Wei-yi, Li Xiao-na, Wang Xiao-jun. Generation and application of three-dimensional cell traction force[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(8): 1301-1306.
share this article
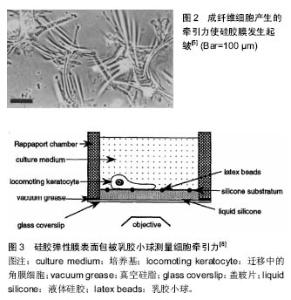
细胞牵张力的测量方法主要有3种:硅胶薄膜测量法(thin silicone membranes)、细胞牵张力显微镜技术(cell traction force microscopy)、微组装悬臂梁(microfabricated cantilevers)[4]。以下将对这3种方法的基本原理及优缺点加以分析。 2.1 硅胶薄膜测量法 牵引力的观测可追溯到20世纪80年代,Harris等[5]将细胞接种在薄的硅胶膜上,发现正在迁移的细胞使硅胶膜产生褶皱(图2)。通过检测产生褶皱的数量及长短发现small GTPase RhoA和钙调蛋白信号途径通过应力纤维和局部黏着斑参与牵张力的调控[6-7]。但这种方法存在以下缺陷:①仅限于具有高度收缩性的几种细胞;②由于褶皱变形的非线性特点,力学分析比较困难[4]。"
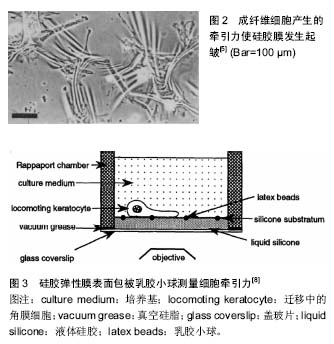

2.2 细胞牵张力显微镜技术 有学者对硅胶薄膜测量法进行了改进,在非起皱硅胶膜弹性膜上包被微米级乳胶小球,细胞产生牵引力使微球的位置发生变化,但并不引起弹性薄膜起皱(图3)[8]。根据小球的位移变化估算牵引力的矢量。这种方法在研究细胞迁移方面做出了一定贡献,但小球的位移和牵引力之间的关系仍为非线性,依旧不能采用弹性理论进行分析。20世纪90年代Oliver等[9]发明了牵张力显微测量(traction force microscope,TFM)技术。该技术能够实现定量分析细胞牵引力的时空分布规律。细胞牵张力显微测量技术的基本原理是将细胞培养在软的凝胶(例如聚丙烯酰胺)或高分子聚合物(例如聚二甲基硅氧烷)弹性基底表面,通过分析计算细胞引起的基底弹性变形信息反演得到细胞牵引力场[10-11]。"
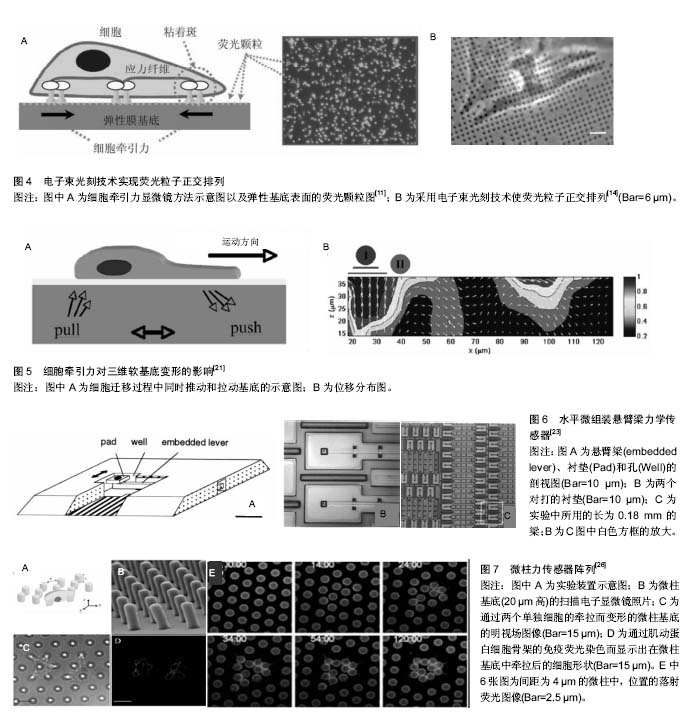
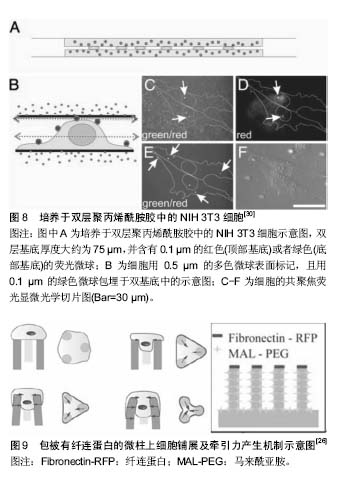
目前,大多数牵张力显微测量技术均假设细胞牵引力引起的基底材料变形为小变形事件,并采用线弹性连续介质理论进行分析。 牵张力显微测量技术的优势在于聚丙烯酰胺凝胶属于线性弹性材料,当施加外力消失后,凝胶的形变可以恢复[12],且在制作胶的过程中可以调控它的弹性模量;根据不同种类的细胞,在胶上表衬所需的细胞外基质蛋白[13]。最初在包埋荧光粒子时相对随机,使粒子分布不够均匀,计算粒子稀少区域的牵引力具有不确定性。电子束光刻技术克服了这种缺陷,可以实现荧光粒子的正交排列(图4)[14]。 牵张力显微测量技术的不足之处在于所用的弹性基底本身会发生变形。局部黏附产生的牵引力会引起一定范围内的基底发生形变,反过来产生一个较弱的力,并作用于临近的黏着斑,使不同位点所产生的牵引力相互影响,无法真正获得局部某个黏附点的牵引力。此外,荧光粒子位移的测量使牵张力显微测量技术的精确度受到一定限制,观测数据的微小偏差可能导致解的很大变动。 牵张力显微测量技术在细胞迁移、细胞-细胞外基质相互作用以及配体密度-细胞铺展与牵引力关系等研究方面做出了很大贡献[12,15-17]。牵张力显微测量技术不仅用于单个细胞牵引力的研究,一些学者还采用有限元分析方法对细胞-细胞以及群体细胞牵引力进行了深入研究[16,18],并结合了数字体积相关技术(digital volume"
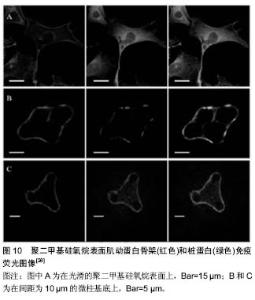
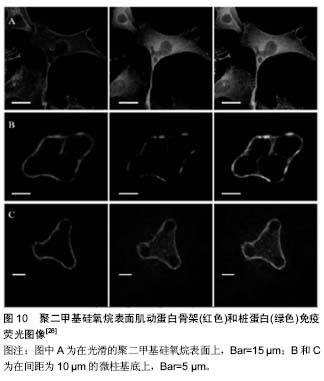
2.3 微组装悬臂梁 如何独立检测到每个黏附点所产生的牵张力?微组装悬臂梁技术应运而生。最初的微组装悬臂梁为水平式,细胞产生的牵张力使悬臂梁顶端发生侧偏,根据悬臂梁刚度与顶端位移即可获得局部牵张力的大小(图6)。有趣的是,采用该方法发现细胞尾部的牵张力要比运动前缘大[23],这与硅胶膜检测结果一致,但与牵张力显微测量技术的检测结果有所不同,提示细胞在迁移过程中运动前缘需要克服来自胞体后部阻力[24]。该方法的不足在于水平悬臂梁是固定的,只能检测到沿一个直线方向的力,而不能提供细胞的二维牵引力图谱。软平板印刷术使该项技术得以改进,催生了垂直微悬臂梁阵列(或称微柱力传感器阵列)技术(图7)。该技术的原理为:细胞牵引力使微柱发生偏转,根据微柱的弯曲变形程度测定细胞牵引力,并能够提供二维牵引力图谱全景。此外,微柱刚度可调,因此,可用其研究细胞在不同刚度微柱上所产生的牵引力[25-26]。采用该方法证实了RhoA信号途径和局部黏着斑参与了牵引力调控,与硅胶薄膜和牵张力显微测量观测到的结果一致[25]。该技术的不足在于微柱是彼此独立的,细胞与基底之间属于非完全接触,因此所提供的微环境不连续,且制作工艺相对复杂。 以上技术主要应用于细胞在二维环境中牵引力的研究,但体内细胞生存的微环境为三维结构,在二维表"
| [1] Discher D, Dong C, Fredberg JJ, et al. Biomechanics: cell research and applications for the next decade. Ann Biomed Eng. 2009;37(5):847-859.[2] Schwarz US, Gardel ML. United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J Cell Sci. 2012;125(Pt 13):3051-3060.[3] Wang JH, Lin JS. Cell traction force and measurement methods. Biomech Model Mechanobiol. 2007;6(6):361-371.[4] Han SJ, Sniadecki NJ. Nanotechnology Usages for Cellular Adhesion and Traction Forces. Cellular and Biomolecular Mechanics and Mechanobiology. 2010; 4:177-200.[5] Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208 (4440):177-179.[6] Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133(6):1403-1415. [7] Helfman DM, Levy ET, Berthier C, et al. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol Biol Cell. 1999;10(10):3097-3112.[8] Lee J, Leonard M, Oliver T, et al. Traction forces generated by locomoting keratocytes. J Cell Biol. 1994;127(6 Pt 2):1957-1964.[9] Oliver T, Dembo M, Jacobson K. Traction forces in locomoting cells. Cell Motil Cytoskeleton. 1995;31(3):225-240.[10] Wang JH, Li B. Application of cell traction force microscopy for cell biology research. Methods Mol Biol. 2009;586:301-313.[11] 黄建永,邓昊,彭小玲,等. 细胞牵引力显微镜反演方法研究进展[J].实验力学,2011,26(5):503-517.[12] Pelham RJ Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94(25):13661-13665.[13] Kandow CE, Georges PC, Janmey PA, et al. Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods Cell Biol. 2007;83:29-46.[14] Balaban NQ, Schwarz US, Riveline D, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466-472.[15] Munevar S, Wang Y, Dembo M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys J. 2001;80(4):1744-1757.[16] Maruthamuthu V, Sabass B, Schwarz US, et al. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A. 2011;108(12):4708-4713.[17] Inoue S, Iida Y, Otani Y, et al. Adhesion behavior of human adipo-stromal cells on self-assembled monolayers with different surface densities or gradients of RGD peptide. J Biomater Sci Polym Ed. 2009;20(4):495-510.[18] Tang X, Tofangchi A, Anand SV, et al. A novel cell traction force microscopy to study multi-cellular system. PLoS Comput Biol. 2014;10(6):e1003631.[19] Notbohm J, Kim JH, Franck C, et al. Three-dimensional Traction Force Microscopy for Studying Cellular Interactions with Biomaterials. Procedia Iutam. 2012; 4(9):144-150.[20] Franck C, Maskarinec SA, Tirrell DA, et al. Three-dimensional traction force microscopy: a new tool for quantifying cell-matrix interactions. PLoS One. 2011;6(3):e17833.[21] Maskarinec SA, Franck C, Tirrell DA, et al. Quantifying cellular traction forces in three dimensions. Proc Natl Acad Sci U S A. 2009;106(52):22108-22113.[22] Toyjanova J, Bar-Kochba E, López-Fagundo C, et al. High resolution, large deformation 3D traction force microscopy. PLoS One. 2014;9(4):e90976.[23] Galbraith CG, Sheetz MP. A micromachined device provides a new bend on fibroblast traction forces. Proc Natl Acad Sci U S A. 1997;94(17):9114-9118.[24] Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8(2):51-54.[25] Tan JL, Tien J, Pirone DM, et al. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100(4):1484-1489.[26] Ghibaudo M, Di Meglio JM, Hersen P, et al. Mechanics of cell spreading within 3D-micropatterned environments. Lab Chip. 2011;11(5):805-812.[27] Cukierman E, Pankov R, Stevens DR, et al. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547): 1708-1712.[28] Doyle AD, Petrie RJ, Kutys ML, et al. Dimensions in cell migration. Curr Opin Cell Biol. 2013;25(5):642-649.[29] Zaman MH, Trapani LM, Sieminski AL, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103(29):10889-10894.[30] Beningo KA, Dembo M, Wang YL. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci U S A. 2004;101(52):18024-18029 |
| [1] | Wang Jianping, Zhang Xiaohui, Yu Jinwei, Wei Shaoliang, Zhang Xinmin, Xu Xingxin, Qu Haijun. Application of knee joint motion analysis in machanism based on three-dimensional image registration and coordinate transformation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-5. |
| [2] | Xu Xinzhong, Wu Zhonghan, Yu Shuisheng, Zhao Yao, Xu Chungui, Zhang Xin, Zheng Meige, Jing Juehua. Biomechanical analysis of different ways of inserting Steinmann Pins into the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1313-1317. |
| [3] | Zhang Yufang, Lü Meng, Mei Zhao. Construction and verification of a full spine biomechanical model of adolescent scoliosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1351-1356. |
| [4] | Bai Zixing, Cao Xuhan, Sun Chengyi, Yang Yanjun, Chen Si, Wen Jianmin, Lin Xinxiao, Sun Weidong. Construction and biomechanical analysis of ankle joint finite element model in gait cycle [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1362-1366. |
| [5] | Liu Feng, Feng Yi. Finite element analysis of different Kirschner wire tension bands on transverse patella fractures during gait cycle [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1367-1371. |
| [6] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [7] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [8] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [9] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [10] | Zhang Yujie, Yang Jiandong, Cai Jun, Zhu Shoulei, Tian Yuan. Mechanism by which allicin inhibits proliferation and promotes apoptosis of rat vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1080-1084. |
| [11] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [12] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [13] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [14] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [15] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||