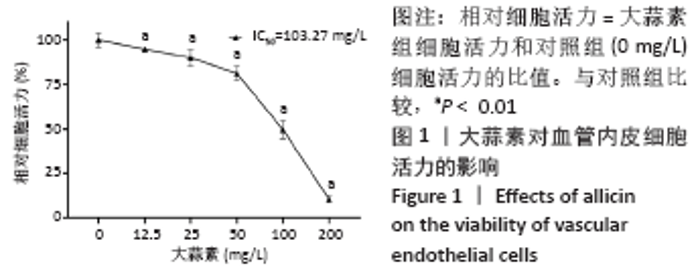
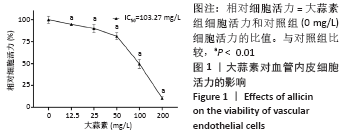
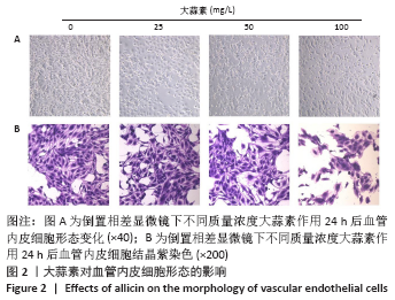
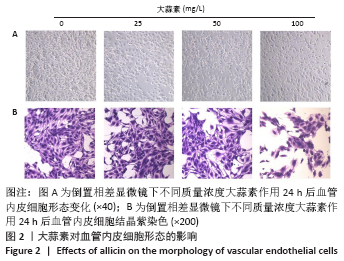
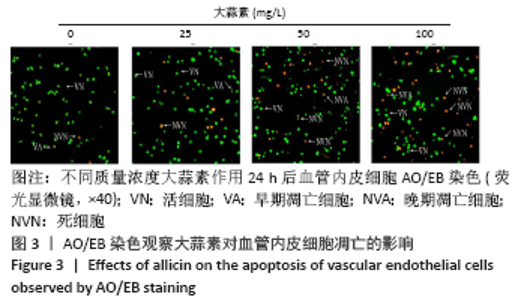
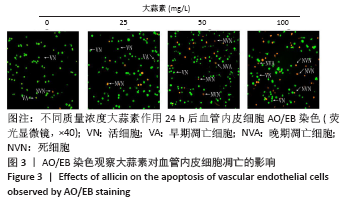
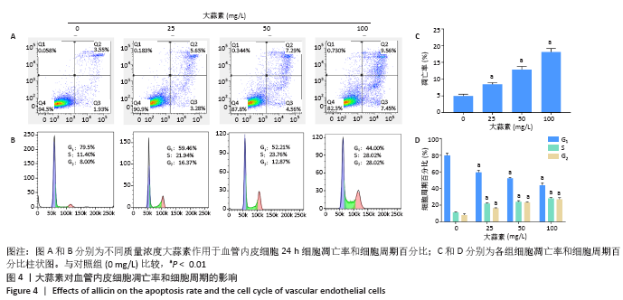
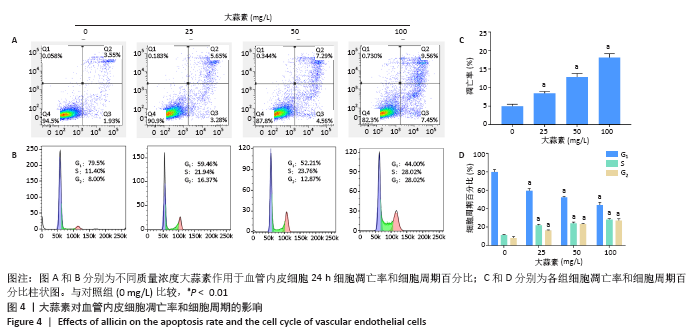
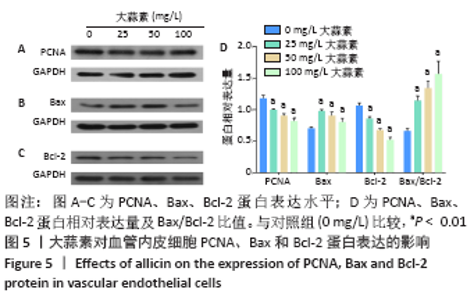
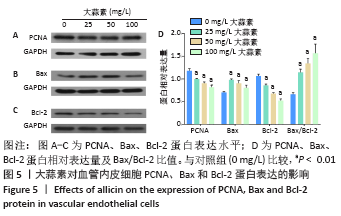
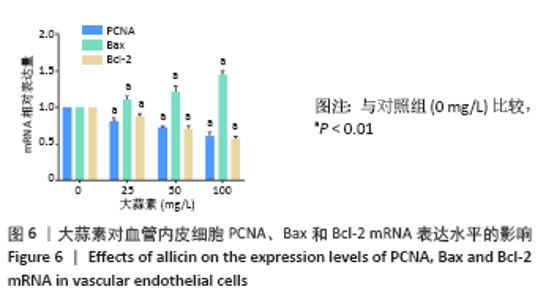
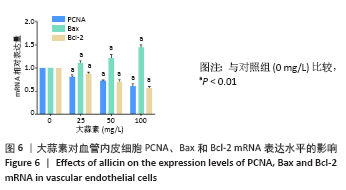
[1] KORNTNER S, LEHNER C, GEHWOLF R, et al. Limiting angiogenesis to modulate scar formation. Adv Drug Deliv Rev. 2019;146:170-189.
[2] BOSSCHER HA, HEAVNER JE. Incidence and severity of epidural fibrosis after back surgery: an endoscopic study. Pain Pract. 2010;10(1):18-24.
[3] COENTRO JQ, PUGLIESE E, HANLEY G, et al. Current and upcoming therapies to modulate skin scarring and fibrosis. Adv Drug Deliv Rev. 2019;146:37-59.
[4] ZENG L, SUN Y, LI X, et al. 10‑Hydroxycamptothecin induces apoptosis in human fibroblasts by regulating miRNA‑23b‑3p expression. Mol Med Rep. 2019;19(4): 2680-2686.
[5] WAN Q, CHEN H, LI X, et al. Artesunate inhibits fibroblasts proliferation and reduces surgery-induced epidural fibrosis via the autophagy-mediated p53/p21waf1/cip1 pathway. Eur J Pharmacol. 2019;842:197-207.
[6] SHI K, WANG F, XIA J, et al. Pirfenidone inhibits epidural scar fibroblast proliferation and differentiation by regulating TGF-β1-induced Smad-dependent and -independent pathways. Am J Transl Res. 2019;11(3):1593-1604.
[7] SERTBAS I, YILMAZ A, YILDIRIM T, et al. The role of pegaptanib sodium in the suppression of epidural fibrosis in a postlaminectomy rat model. Bratisl Lek Listy. 2017;118(2):118-122.
[8] BORLINGHAUS J, ALBRECHT F, GRUHLKE MC, et al. Allicin: chemistry and biological properties. Molecules. 2014;19(8):12591-12618.
[9] SUN HH, WANG JC, FENG XM, et al. Allicin Inhibits Proliferation and Promotes Apoptosis of Human Epidural Scar Fibroblasts. World Neurosurg. 2020;136:e460-e468.
[10] 李希敏,徐露,周璐,等.大蒜素对大鼠肾间质纤维化干预作用及对肾组织TGF-β1/Smads信号通路的影响[J].浙江中西医结合杂志,2019,29(10):793-796.
[11] 陆晓丹,宗刚军,周建英,等.大蒜素改善糖尿病心肌病大鼠心功能及纤维化的作用及对NF-κB信号通路的影响[J].中国药业,2019,28(13):22-25.
[12] 朱守雷,杨建东,蔡俊,等.大蒜素抑制成纤维细胞增殖、迁移并诱导其凋亡的机制[J].中国组织工程研究,2020,24(35):5662-5667.
[13] RABB CH. Failed back syndrome and epidural fibrosis. Spine J. 2010;10(5):454-455.
[14] CHO JH, LEE JH, SONG KS, et al. Neuropathic Pain after Spinal Surgery. Asian Spine J. 2017;11(4):642-652.
[15] WANG QJ, ZHANG AL, KANG ZQ, et al. Exogenous IL-19 mediates downregulation of TGF-β through Erk and p38 pathway to inhibit epidural fibrosis. Eur Rev Med Pharmacol Sci. 2019;23(17):7184-7190.
[16] EMING SA, BRACHVOGEL B, ODORISIO T, et al. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007;42(3):115-170.
[17] TONNESEN MG, FENG X, CLARK RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5(1):40-46.
[18] SZPADERSKA AM, WALSH CG, STEINBERG MJ, et al. Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res. 2005;84(4):309-314.
[19] SONG Y, YU Z, SONG B, et al. Usnic acid inhibits hypertrophic scarring in a rabbit ear model by suppressing scar tissue angiogenesis. Biomed Pharmacother. 2018; 108:524-530.
[20] DIPIETRO LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100(5):979-984.
[21] SHANG A, CAO SY, XU XY, et al. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods. 2019;8(7):246.
[22] BLOCK E, BECHAND B, GUNDALA S, et al. Fluorinated Analog NMR s of Organosulfur Compounds from Garlic (Allium sativum): Synthesis, Chemistry and Anti-Angiogenesis and Antithrombotic Studies. Molecules. 2017;22(12):2081.
[23] SUN HH, FENG XM, WANG JC, et al. Allicin can suppress the activity of vascular endothelial cells probably by regulating JAK2/STAT3 pathway. Mol Cell Biochem. 2020 Sep 25. doi: 10.1007/s11010-020-03919-z. Online ahead of print.
[24] KRÜGER-GENGE A, BLOCKI A, FRANKE RP, et al. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci. 2019;20(18):4411.
[25] PRELICH G, TAN CK, KOSTURA M, et al. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987; 326(6112):517-520.
[26] SAEEDI BORUJENI MJ, HAMI J, HAGHIR H, et al. Evaluation of Bax and Bcl-2 Proteins Expression in the Rat Hippocampus due to Childhood Febrile Seizure. Iran J Child Neurol. 2016;10(1):53-60.
[27] RENAULT TT, DEJEAN LM, MANON S. A brewing understanding of the regulation of Bax function by Bcl-xL and Bcl-2. Mech Ageing Dev. 2017;161(Pt B):201-210.
[28] BUSCH C, JACOB C, ANWAR A, et al. Diallylpolysulfides induce growth arrest and apoptosis. Int J Oncol. 2010;36(3):743-749.
[29] KELKEL M, CERELLA C, MACK F, et al. ROS-independent JNK activation and multisite phosphorylation of Bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis. 2012;33(11):2162-2171.
[30] GRUHLKE MC, NICCO C, BATTEUX F, et al. The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines. Antioxidants (Basel). 2016;6(1):1.
[31] WANG SL, LIU DS, LIANG ES, et al. Protective effect of allicin on high glucose/hypoxia-induced aortic endothelial cells via reduction of oxidative stress. Exp Ther Med. 2015;10(4):1394-1400.
|