Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (28): 4243-4249.doi: 10.3969/j.issn.2095-4344.2016.28.020
Previous Articles Next Articles
Traditional Chinese medicine in stem cell proliferation and tissue engineering
Zhang Ye1, 2, Cui Yuan-lu2, 3
- 1Zibo Vocational College, Zibo 255314, Shandong Province, China
2 Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China
3Tianjin State Key Laboratory of Modern Chinese Medicine (State Key Laboratory), Tianjin 300193, China
-
Revised:2016-06-07Online:2016-07-01Published:2016-07-01 -
About author:Zhang Ye, M.D., Lecturer, Zibo Vocational College, Zibo 255314, Shandong Province, China; Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China -
Supported by:the National Natural Science Foundation of China, No. 30973967; the Research Fund for the Doctoral Program of Higher Education of Chinese Ministry of Education, No. 20091210110003; the Scientific and Technological Xinghai Plan Project of Tianjin, No. KX2010-0005
CLC Number:
Cite this article
Zhang Ye, Cui Yuan-lu. Traditional Chinese medicine in stem cell proliferation and tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(28): 4243-4249.
share this article

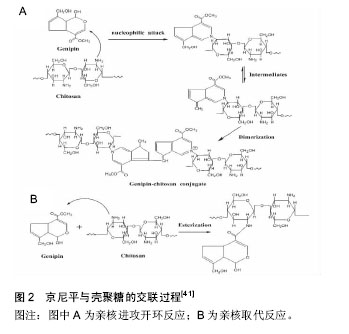
2.1 诱导干细胞增殖和分化 骨髓间充质干细胞是骨髓中的一类干细胞,具有多向分化潜能,取材方便,体外培养能迅速扩增,体内植入反应较弱,与宿主神经组织能良好整合并长期存活等优势。骨髓间充质干细胞不但可以向成骨、软骨细胞分化,还能向神经细胞、心肌细胞等分化。 黎晖等[5]报道了龟板体外成功诱导骨髓间充质干细胞向成骨细胞分化,诱导后细胞出现成纤维细胞外观等形态学变化,成骨细胞重要标志物碱性磷酸酶活性、钙化结节、骨钙素的含量明显升高,与对照组比较差异有显著性意义(P < 0.05)。吴追乐等[6]表明透骨消痛颗粒含药血清能增强Ⅱ型胶原在细胞分化过程中的表达,诱导分化作用机制可能是全方位、多靶点的复杂网络调控。肖庆忠等[7]采用含有麝香多肽的无血清L-DMEM培养基体外诱导大鼠骨髓间充质干细胞,发现在加入该培养基后,细胞胞体收缩,突起伸出,形似神经元,免疫组化检测神经干细胞特异性标志(nestin)、神经元特异性标志(神经元特异性烯醇化酶和神经丝蛋白)阳性,星形胶质细胞特异性标志(胶质纤维酸性蛋白)阴性;而在相同条件用含有麝香酮的无血清L-DMEM培养基对基质细胞进行诱导却没有上述变化,可见麝香多肽能体外诱导大鼠骨髓间充质干细胞分化为神经元样细胞,而麝香酮则不能。李志泉等[8]报道三七总皂苷促进大鼠骨髓间充质干细胞增殖,并可体外诱导骨髓间充质干细胞分化为心肌样细胞。加入三七总皂苷后大鼠骨髓间充质干细胞表面抗原CD44阳性,CD34阴性;经三七总皂苷作用后骨髓间充质干细胞增长速度比空白对照组明显加快,体外诱导后细胞形态发生变化,其细胞免疫组化鉴定呈结蛋白(Desmin)、肌钙蛋白(cTnI)阳性;RT-PCR检测结果显示诱导后细胞转录肌球蛋白重链mRNA水平增加,说明三七总皂苷能促进大鼠骨髓间充质干细胞体外增殖并可诱导分化为心肌样细胞。 此外,中药可成功促进胚胎干细胞、造血干细胞、脐血间充质干细胞、脂肪源性干细胞等增殖和分化。陈晓岚等[9]用中药黄芩有效成分黄芩苷体外成功诱导人脐血间充质干细胞分化为神经元样细胞,低浓度的黄芩苷体外扩增2周后的脐血间充质干细胞中已有少量表达神经元特异性烯醇化酶的阳性细胞,这在一定程度上起到了预诱导分化作用。诱导过程中黄芩苷的诱导作用温和、稳定而持久,诱导出的神经元样细胞成活时间长,他们认为其诱导机制可能与黄芩苷的抗氧化、调控细胞NF-κB活性从而刺激多种细胞因子的表达和生成有关。周琦等[10]分别采用常规培养液、成软骨诱导液及成软骨诱导液加丹参注射液培养C57小鼠脂肪源性干细胞14 d,定量PCR检测表明诱导组和丹参注射液组均可促进脂肪源性干细胞向软骨细胞分化,与诱导组相比,丹参注射液组可以提高CollagenⅡmRNA的表达水平。2月龄裸鼠软骨缺损实验表明丹参注射液组的软骨再生功能强于诱导组。 2.2 抗炎 粉防己碱是从防己中提取的一种双苄基异喹啉类生物碱,具有良好的抗炎和免疫抑制作用,可以抑制炎症过程的多个环节,包括抑制炎症细胞功能、抗自由基损伤、抑制炎症递质释放和对抗炎症递质的效应、抗血小板聚集。低浓度的粉防己碱可以促进软骨细胞增殖、提高软骨细胞活性、维持软骨细胞的功能表达,有利于体外软骨组织重建[11]。根据粉防己碱的药理作用,崔元璐等[12]将粉防己碱负载于聚乳酸薄膜中,利用粉防己碱的弱碱性和抗炎药理活性缓解聚乳酸降解过程中造成的局部pH变化刺激,防止无菌性炎症的发生。结果表明,软骨细胞在含有粉防己碱的聚乳酸膜和单纯聚乳酸膜表面的行为有明显的差异。含有低浓度粉防己碱的聚乳酸膜(0.04 mg/g,0.1 mg/g)可以促进载药材料表面贴附生长的软骨细胞增殖,提高软骨细胞活性,增强细胞功能的表达(提高糖胺聚糖和Ⅱ型胶原分泌量)。随后,他们采用体内外实验的方法验证载粉防己碱聚乳酸膜的抗炎活性[13]。结果表明粉防己碱的载入对聚乳酸的表面接触角、热力学特征、表面形貌和非晶态结构没有产生影响。体外构建了IFN-γ诱导的小鼠单核-巨噬细胞株RAW264.7细胞非菌性细胞炎症模型,载粉防己碱的聚乳酸膜材料可显著降低一氧化氮、肿瘤坏死因子α、白细胞介素6、诱导型一氧化氮合成酶、COX-2的表达水平,这表明载药聚乳酸膜可通过调节mRNA和蛋白表达降低巨噬细胞的炎症应答;大鼠体内埋置实验表明,在植入4周和12周后,载药聚乳酸膜的恢复程度显著优于空白聚乳酸膜。这预示了载粉防己碱的生物材料可作为软骨组织体外修复重建的支架材料和软骨组织工程支架材料,有着良好的应用前景。 2.3 促进成骨细胞增殖与分化 淫羊藿,功效补肾壮阳、强筋健骨、祛风除湿,可促进骨愈合而用于骨质疏松的治疗。Zhang等[14]证实淫羊藿中的黄酮类成分可同时促进骨髓间充质干细胞成骨分化和抑制骨髓间充质干细胞破骨分化。淫羊藿苷(C33H40O15)是淫羊藿的主要有效成分之一,作为一种骨合成因子,通过雌激素样作用,调控OPG/RANK/RANKL系统[15],调控转录因子OSX[16]、MAPK通路[17]、BMP-2/Smad4信号转导通路[18-20]、血管内皮生长因子[21],诱导Cbfa1/Runx2、OPG、RANKL的表达[19],促进骨形态发生蛋白4的产生和一氧化氮的合成[19-20],促进成骨细胞增殖与分化;并且通过p38和JNK信号通路阻止脂多糖诱导的破骨细胞生成[22]。根据淫羊藿苷的成骨性能,Zhao等[23]将磷酸钙骨水泥负载淫羊藿苷,填充于小鼠颅骨缺损中,颅骨标本显示明显的新骨和血管生成,骨厚度增加。将淫羊藿苷负载于多孔β-磷酸三钙陶瓷片中,药物的加入并未影响陶瓷片本身的形态,并且含有药物的陶瓷片具有良好的细胞相容性,可使细胞增殖和分化维持在比较高的水平。将含有药物的多孔β-磷酸三钙陶瓷片植入大鼠背部肌肉下,可见明显的骨生成,而不含淫羊藿苷的陶瓷片未见明显骨生成[24]。 2.4 表面修饰 移植物的表面性质对细胞的黏附和生长有很大的影响,因此对生物材料进行表面修饰是构建组织工程的重要环节。研究已证明相对于空白左旋聚乳酸膜,黄芩苷修饰的左旋聚乳酸膜可明显增强颅骨成骨细胞的附着能力和增殖能力,显著提高细胞活性和碱性磷酸酶活性,这表明经黄芩苷修饰可显著提高左旋聚乳酸膜的生物相容性[25]。随后,他们采用物理截留法使得黄芩苷修饰3D左旋聚乳酸支架表面,用兔桡骨损伤模型对其进行体内组织学评价。结果表明相对空白支架,黄芩苷修饰的左旋聚乳酸支架能明显促进成骨细胞产生骨钙蛋白,有利于成骨细胞分化,从而促进骨形成。组织学观察及组织形态学皆表明黄芩苷修饰的左旋聚乳酸支架具有较高的促进骨形成的能力和较好的生物相容性[26]。 2.5 促进血管形成 丹参酮ⅡA是丹参的主要有效成分之一,具有天然的抗氧化作用,表现为抗动脉粥样硬化,缩小心肌梗死面积,降低心肌耗氧量,对血栓形成以及血小板聚集功能有抑制作用[27];对肿瘤细胞具有杀伤功能,诱导分化和凋亡等作用[28]。基于丹参酮ⅡA的药理作用和临床实践,Zhao等[29]制备了载有丹参酮ⅡA的壳聚糖-明胶膜,其体外释放符合Higuchi方程,且随载药量增大药物释放速率加快,壳聚糖-明胶膜(1︰2)15 d仅释放20%,体内降解实验表明28 d内膜可完全降解,并且植入7 d后大鼠腹腔伤口愈合。三七作为一种常见的活血化瘀中药具有良好的促血管生成作用。郑玉蓉等[30]研究表明三七促进血管生成的主要机制为三七皂甙Rg1增加人脐静脉血管内皮细胞的G2/M期和S期比例,并未引起内皮细胞的凋亡;同时上调人脐静脉血管内皮细胞血管内皮生长因子表达。随后,他们将三七皂甙Rg1负载于胶原-壳聚糖-明胶微球(CC-GMS)支架中,体内外实验研究该支架促血管生成的作用。结果表明胶原-壳聚糖-明胶微球支架具有良好的生物相容性,能对有效成分三七皂甙Rg1进行缓释,并能促进SD大鼠皮肤血管化生成。陈述祥等[31]也证实了载有三七总皂苷的支架具有良好的生物相容性。他们采用原位复合和冷冻干燥技术制备了三七总皂苷-羟基磷灰石/壳聚糖骨修复支架,研究表明药物的载入对支架的孔隙率、孔径、密度无显著影响,但却降低了其断裂强度和弹性模量,载药量越大效果越明显。载药前后的复合材料均具有良好的血液相容性。 2.6 抗凝血 药物洗脱支架具有良好的抗增生性能,对血管支架内再狭窄起到很好的抑制作用,但其抗凝血性能仍有待改善。唐家驹等[32]以可降解高分子材料聚乳酸为载体制备了质量分数为3%、5%的大黄素复合薄膜。体外血小板黏附实验和部分凝血活酶时间表征复合薄膜的体外抗凝血性能。结果显示复合薄膜的血小板黏附和聚集数量减少且部分凝血活酶时间长于纯聚乳酸薄膜,因此大黄素的载入有效地改进了聚乳酸薄膜的抗凝血性能。陈艳等[33]将姜黄素负载于聚乳酸-羟基乙酸共聚物支架中,实验结果也证实了含有药物的支架具有良好的抗凝血性能,且抗凝血性能随着姜黄素含量的增大而提高。 2.7 交联 传统的化学交联剂戊二醛、碳化二亚胺等本身具有相对较高的细胞毒性,导致所得的生物材料在植入受体以后,会影响正常组织的生长,而且在体内不易降解,机械性能也不理想。而环烯醚萜类京尼平,多酚类原花青素、单宁酸等中药有效成分作为天然交联剂能够克服传统化学交联剂的缺点,逐渐取而代之,在生物医学材料的改性方面发挥更大的作用。 京尼平化学结构中具有羟基、羧基等多个活性基团,通过亲核进攻开环反应和双分子亲核取代反应与明胶、壳聚糖等含有自由氨基的化合物发生环状交联反应[34],生成叔胺结构;这种结构相对于戊二醛与自由氨基反应生成的Schiff碱结构更加稳定,因此京尼平交联的材料与戊二醛相比,具有更低的溶胀率和更好的热稳定性[35]。京尼平作为交联剂还具有细胞毒性低、生物相容性好、抗降解性好、机械性能高等特点。在人的创伤皮肤修复实验中,Chang等[36]用不同的交联剂处理伤口敷料,手术14 d后发现,用戊二醛交联的敷料伤口中央仅有真皮,并且存在许多炎症细胞,而京尼平交联的敷料伤口处已长满了真皮和表皮,伤口边缘的炎症细胞也完全消失。Lien等[37]采用凝胶化-交联-冷冻干燥制得了孔径较小且均匀的明胶支架,对Wistar大鼠软骨细胞进行实验。结果显示京尼平交联的明胶支架对细胞无毒性,9 d内会产生胶原和糖胺聚糖,30 d内会长成与天然软骨相似的细胞分布。但是京尼平的低毒性具有一定的种属和细胞特异性,其在较低浓度下对L929 成纤维细胞[38]、猪软骨细胞[39]、牛软骨细胞等有明显毒性[40]。而且京尼平价格比较贵,这些都限制了京尼平的进一步应用。京尼平与壳聚糖的交联过程见图2。"
| [1] Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920-926. [2] Friess W. Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113-136. [3] Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946-1949. [4] Sun JS, Dong GC, Lin CY, et al. The effect of Gu-Sui-Bu (Drynaria fortunei J. Sm) immobilized modified calcium hydrogenphosphate on bone cell activities. Biomaterials. 2003;24(5):873-882. [5] 黎晖,周健洪,陈东风,等. 龟板对大鼠骨髓间充质干细胞向成骨分化的影响[J].中药新药与临床药理,2005, 16(3): 159-161. [6] 吴追乐,刘献祥,李西海,等.透骨消痛颗粒诱导骨髓间充质干细胞向软骨细胞的分化[J].中国组织工程与临床康复, 2009,13(33):6456-6460. [7] 肖庆忠,温冠媚,李浩威,等. 麝香组分诱导成年大鼠骨髓间质干细胞体外定向分化为神经元样细胞的能力[J].中山医科大学学报,2002,23(6):405-408. [8] 李志泉,冼绍祥,汪朝晖,等.三七总皂苷对骨髓间充质干细胞增殖和向心肌样细胞分化的影响[J].广州中医药大学学报, 2007,24(6):470-475. [9] 陈晓岚,黄仁彬,莫艳秀,等.黄芩甙诱导人脐血间充质干细胞分化为神经元样细胞[J].中风与神经疾病杂志,2007, 24(3):278-281. [10] 周琦,王君,魏义勇.丹参注射液对C57小鼠脂肪源性干细胞成软骨的促进作用[J].上海中医药大学学报,2014, 28(1):64-67. [11] 崔元璐,姚康德,戚爱棣,等.粉防己碱对体外培养软骨细胞的影响[J].中草药,2006,37(12):1847-1850. [12] 崔元璐,戚爱棣,李克峰,等.含粉防己碱生物材料对体外培养软骨细胞的影响[J].天津中医药,2005,22(3):236-239. [13] Wang QS, Cui YL, Gao LN, et al. Reduction of the pro-inflammatory response by tetrandrine-loading poly(L-lactic acid) films in vitro and in vivo. J Biomed Mater Res A. 2014;102(11):4098-4107. [14] Zhang JF, Li G, Meng CL, et al. Total flavonoids of Herba Epimedii improves osteogenesis and inhibits osteoclastogenesis of human mesenchymal stem cells. Phytomedicine. 2009;16(6-7):521-529. [15] 张秀珍,杨黎娟. 淫羊藿甙对大鼠成骨细胞护骨素、RANKL表达的影响[J].中华内分泌代谢杂志,2006,22(3): 222-225. [16] 李淑梅,宋利格,张秀珍.淫羊藿甙对大鼠成骨细胞功能及Osterix表达的影响[J].同济大学学报:医学版,2005,26(6): 8-11. [17] 刘尚全,杨颖,周丽斌,等.淫羊藿甙逆转地塞米松抑制成骨细胞分化及其机制[J].中华内分泌代谢杂志,2006,22(3): 218-221. [18] Liang W, Lin M, Li X, et al. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int J Mol Med. 2012;30(4):889-895. [19] Zhao J, Ohba S, Shinkai M, et al. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem Biophys Res Commun. 2008;369(2):444-448. [20] Hsieh TP, Sheu SY, Sun JS, et al. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine. 2010;17(6):414-423. [21] 程杰,王永清,彭锐,等.生骨注射液对大鼠成骨细胞VEGF mRNA表达的影响[J].中国矫形外科杂志,2005,13(5): 362-364. [22] Hsieh TP, Sheu SY, Sun JS, et al. Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis. Phytomedicine. 2011;18(2-3): 176-185. [23] Zhao J, Ohba S, Komiyama Y, et al. Icariin: a potential osteoinductive compound for bone tissue engineering. Tissue Eng Part A. 2010;16(1):233-243. [24] Zhang X, Guo Y, Li DX, et al. The effect of loading icariin on biocompatibility and bioactivity of porous β-TCP ceramic. J Mater Sci Mater Med. 2011;22(2):371-379. [25] Liu WG, Li XW, Li YS, et al. Effects of baicalin-modified poly(D,L-lactic acid) surface on the behavior of osteoblasts. J Mater Sci Mater Med. 2003;14(11): 961-965. [26] Cai K, Yao K, Yang Z, et al. Surface modification of three-dimensional poly(d,l-lactic acid) scaffolds with baicalin: a histological study. Acta Biomater. 2007;3(4): 597-605. [27] Huang H, Tang Y, Zhang C. The study of committed differentiation from adult rMSCs into neuron-like cells induced by Xiangdan injection in vitro. Zhong Yao Cai. 2004;27(8):585-589. [28] Wu Y, Yang Y, Meng W, et al. Study on the differentiation of K562 cell-line induced by Tanshinone II A. Hua Xi Yi Ke Da Xue Xue Bao. 2002;33(1):80-83. [29] Zhao HR, Wang K, Zhao Y, et al. Novel sustained-release implant of herb extract using chitosan. Biomaterials. 2002;23(23):4459-4462. [30] 郑玉蓉. 胶原/壳聚糖—明胶微球支架缓释三七皂甙Rg1促血管化研究[D].杭州:浙江大学,2014. [31] 陈述祥,康乐,区文欢,等. 载中药羟基磷灰石/壳聚糖骨支架的制备与生物相容性[J].中国组织工程研究,2013, 17(34):6116-6122. [32] 唐家驹,潘长江,洪玮,等.大黄素/聚乳酸物复合薄膜的制备及抗凝血研究[J].功能材料,2006,37(8):1312-1313. [33] 陈艳,林杰,张晓静,等.静电纺PLGA/姜黄素复合薄膜的制备及抗凝血研究[J].材料导报, 2010,24(12):98-100. [34] Nickerson MT, Patel J, Heyd DV, et al. Kinetic and mechanistic considerations in the gelation of genipin- crosslinked gelatin. Int J Biol Macromol. 2006;39(4-5): 298-302. [35] Bigi A, Cojazzi G, Panzavolta S, et al. Stabilization of gelatin films by crosslinking with genipin. Biomaterials. 2002;23(24):4827-4832. [36] Chang WH, Chang Y, Lai PH, et al. A genipin-crosslinked gelatin membrane as wound-dressing material: in vitro and in vivo studies. J Biomater Sci Polym Ed. 2003; 14(5): 481-495. [37] Lien SM, Li WT, Huang TJ. Genipin-crosslinked gelatin scaffolds for articular cartilage tissue engineering with a novel crosslinking method. Materials Science and Engineering: C. 2008;28(1):36-43. [38] Sundararaghavan HG, Monteiro GA, Lapin NA, et al. Genipin-induced changes in collagen gels: correlation of mechanical properties to fluorescence. J Biomed Mater Res A. 2008;87(2):308-320. [39] Wang C, Lau TT, Loh WL, et al. Cytocompatibility study of a natural biomaterial crosslinker--Genipin with therapeutic model cells. J Biomed Mater Res B Appl Biomater. 2011;97(1):58-65. [40] Lima EG, Tan AR, Tai T, et al. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res A. 2009;91(3):692-700. [41] Chen H, Ouyang W, Lawuyi B, et al. Reaction of chitosan with genipin and its fluorogenic attributes for potential microcapsule membrane characterization. J Biomed Mater Res A. 2005;75(4):917-927. [42] Hagerman AE, Klucher KM. Tannin-protein interactions. Prog Clin Biol Res. 1986;213:67-76. [43] Dongmo AB, Kamanyi A, Anchang MS, et al. Anti-inflammatory and analgesic properties of the stem bark extracts of Erythrophleum suaveolens (Caesalpiniaceae), Guillemin & Perrottet. J Ethnopharmacol. 2001;77(2-3):137-141. [44] Shahat AA, Cos P, De Bruyne T, et al. Antiviral and antioxidant activity of flavonoids and proanthocyanidins from Crataegus sinaica. Planta Med. 2002;68(6):539-541. [45] Zhai W, Chang J, Lin K, et al. Crosslinking of decellularized porcine heart valve matrix by procyanidins. Biomaterials. 2006;27(19):3684-3690. [46] Isenburg JC, Simionescu DT, Vyavahare NR. Elastin stabilization in cardiovascular implants: improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials. 2004;25(16):3293-3302. [47] Konno K, Hirayama C, Yasui H, et al. Enzymatic activation of oleuropein: a protein crosslinker used as a chemical defense in the privet tree. Proc Natl Acad Sci U S A. 1999;96(16):9159-9164. [48] Madhan B, Subramanian V, Rao JR, et al. Stabilization of collagen using plant polyphenol: role of catechin. Int J Biol Macromol. 2005;37(1-2):47-53. [49] Chen CN, Sung HW, Liang HF, et al. Feasibility study using a natural compound (reuterin) produced by Lactobacillus reuteri in sterilizing and crosslinking biological tissues. J Biomed Mater Res. 2002;61(3): 360-369. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [5] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [6] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [7] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [8] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [9] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [10] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [11] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [12] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [13] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [14] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [15] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||