Chinese Journal of Tissue Engineering Research ›› 2014, Vol. 18 ›› Issue (1): 88-93.doi: 10.3969/j.issn.2095-4344.2014.01.015
Previous Articles Next Articles
Amplification of rabbit adipose-derived stem cells using explants culture method
Liu Qin, Wang Li-ping, Yu Jing, Chen Fang, Diao Bo, Zhang Yi
- Department of Medical Laboratory, Wuhan General Hospital of Guangzhou Military Command, Wuhan 430070, Hubei Province, China
-
Online:2014-01-01Published:2014-01-01 -
Contact:Zhang Yi, Master, Chief pharmacist, Department of Medical Laboratory, Wuhan General Hospital of Guangzhou Military Command, Wuhan 430070, Hubei Province, China -
About author:Liu Qin, Master, Technician, Department of Medical Laboratory, Wuhan General Hospital of Guangzhou Military Command, Wuhan 430070, Hubei Province, China
CLC Number:
Cite this article
Liu Qin, Wang Li-ping, Yu Jing, Chen Fang, Diao Bo, Zhang Yi . Amplification of rabbit adipose-derived stem cells using explants culture method[J]. Chinese Journal of Tissue Engineering Research, 2014, 18(1): 88-93.
share this article
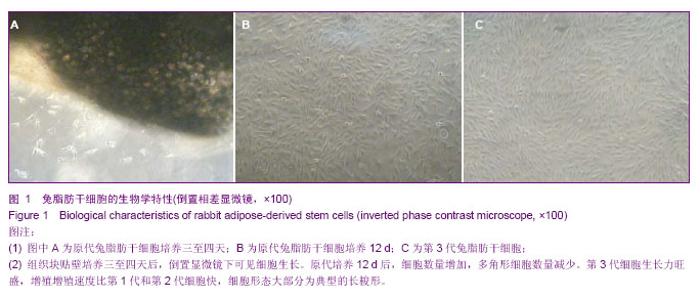
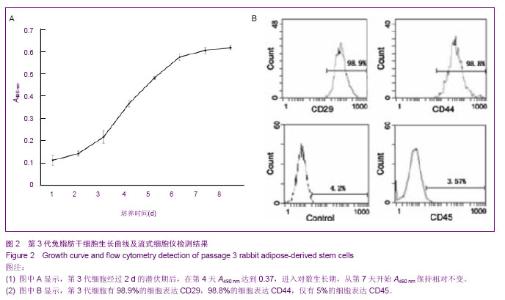
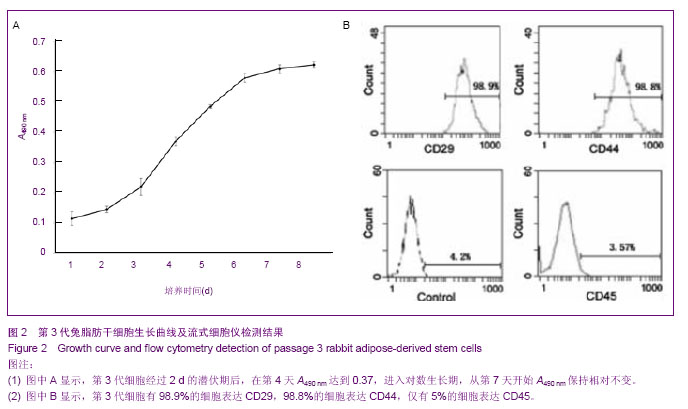
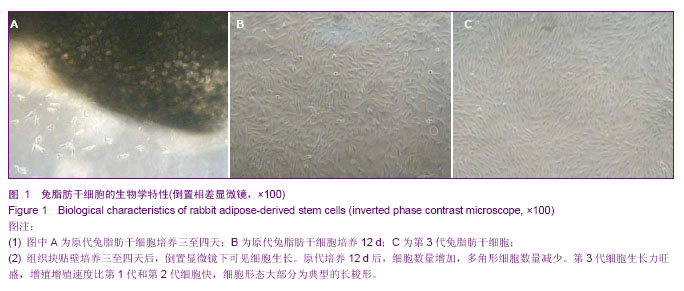
2.1 兔脂肪干细胞的生物学特性 组织块接种2 d后,在光镜下可见较少的细胞从组织块中移行出来,形态多为梭形,少数为圆形。贴壁后三至四天,在光镜下可见更多的细胞从组织块中移行出来,细胞大小较均一,形态多为典型的长梭形,偶见多角形,向四周呈单层贴壁生长(图1A)。7 d时去除组织块。12 d后,细胞数量增多,形态变为均一的梭形,达到80%左右的融合(图1B)。 细胞传至P3时,可见大量成纤维细胞样细胞均匀分布,呈明显的旋涡状生长(图1C),初步判断这些贴壁生长的成纤维样细胞就是兔脂肪干细胞。 四五天传代1次,可长期稳定传代。传代后大部分细胞五至六小时贴壁,12-24 h开始生长,4 d左右达到80%-90%融合。"
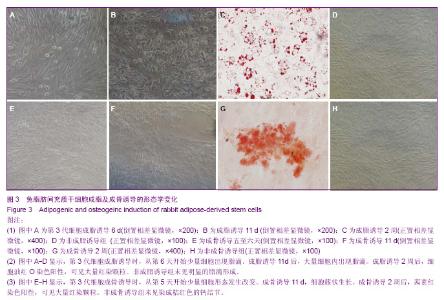
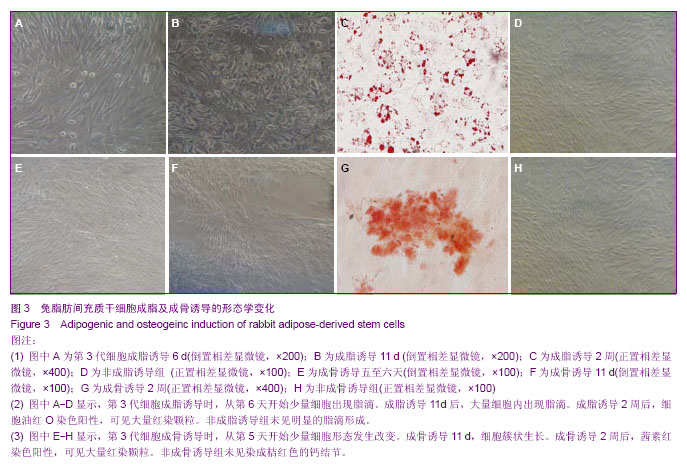
2.4 成脂诱导分化 为鉴定组织块贴壁法培养获得的细胞向脂肪细胞的分化能力,取P3细胞,做成细胞爬片,加入成脂分化诱导液培养,诱导3 d后少量细胞形态由长梭形逐渐变为多角形、不规则形。诱导6 d后胞浆内出现少量脂滴(图3A)。11 d时细胞形态变为明显的多角形、不规则形,细胞内出现大量的脂滴(图3B)。2周时小心吸弃诱导培养液并用40 g/L多聚甲醛固定后加入油红O染色,细胞内出现大量红染颗粒(图3C)。对照组细胞无明显变化(图3D)。表明获得的细胞可被诱导向脂肪细胞分化。 2.5 成骨诱导分化 为鉴定组织块贴壁法培养获得的细胞向成骨细胞的分化能力,取P3细胞,做成细胞爬片,加入成骨分化诱导液培养,诱导五至六天后,可见细胞部分形态由长梭形变为多角形、不规则形、类圆形(图3E)。11 d后可见多角形、不规则形细胞堆积,呈明显的簇状生长(图3F)。2周时小心吸弃诱导培养液并用40 g/L多聚甲醛固定后加入茜素红染色可见桔红色的钙结节(图3G)。对照组细胞无明显变化(图3H)。表明获得的细胞可被诱导向成骨细胞分化。"
| [1] de Girolamo L, Arrigoni E, Stanco D, et al. Role of autologous rabbit adipose-derived stem cells in the early phases of the repairing process of critical bone defects. J Orthop Res. 2011; 29(1):100-108.[2] Arrigoni E, de Girolamo L, Di Giancamillo A, et al. Adipose-derived stem cells and rabbit bone regeneration: histomorphometric, immunohistochemical and mechanical characterization. J Orthop Sci. 2013;18(2):331-339.[3] Li H, Xu Y, Fu Q, et al. Effects of multiple agents on epithelial differentiation of rabbit adipose-derived stem cells in 3D culture. Tissue Eng Part A. 2012;18(17-18):1760-1770.[4] Arrigoni E, de Girolamo L, Di Giancamillo A, et al. Adipose-derived stem cells and rabbit bone regeneration: histomorphometric, immunohistochemical and mechanical characterization. J Orthop Sci. 2013;18(2):331-339.[5] Baptista LS, do Amaral RJ, Carias RB, et al. An alternative method for the isolation of mesenchymal stromal cells derived from lipoaspirate samples. Cytotherapy. 2009;11(6):706-715. [6] 彭智,陈崎,贾振华,等.组织块培养法扩增人脂肪源性干细胞的生物学特征鉴定[J].中国组织工程研究, 2010, 14(36):6689-6694.[7] Sunay O, Can G, Cakir Z, et al. Autologous rabbit adipose tissue-derived mesenchymal stromal cells for the treatment of bone injuries with distraction osteogenesis. Cytotherapy. 2013;15(6):690-702.[8] Sgodda M, Aurich H, Kleist S, et al. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313(13):2875-2886.[9] Toghraie F, Razmkhah M, Gholipour MA, et al. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012; 15(8): 495-499.[10] 庞荣清,何洁,李福兵,等.一种简单的人脐带间充质干细胞分离培养方法[J].中华细胞与干细胞杂志:电子版,2011,1(2):162-167.[11] Yang X, Qu L, Wang X, et al. Plasticity of epidermal adult stem cells derived from adult goat ear skin. Mol Reprod Dev. 2007;74(3):386-396.[12] Spath L, Rotilio V, Alessandrini M, et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J Cell Mol Med. 2010;14(6B):1635- 1644.[13] Priya N, Sarcar S, Majumdar AS, et al. Explant culture: a simple, reproducible, efficient and economic technique for isolation of mesenchymal stromal cells from human adipose tissue and lipoaspirate. J Tissue Eng Regen Med. 2012; 1-9.[14] Ishige I, Nagamura-Inoue T, Honda MJ, et al. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton's jelly explants of human umbilical cord. Int J Hematol. 2009;90(2):261-269.[15] Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110(3):349-355.[16] Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and luid portions of liposuction aspirates. J Cell Physiol. 2006;208(1): 64-76.[17] Brzoska M, Geiger H, Gauer S, et al. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330(1):142-150.[18] Sotiropoulou PA, Perez SA, Salagianni M, et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24(2):462-471.[19] Ou Y, Yuan XD, Cai YN, et al. A novel ethanol-based method to induce differentiation of adipose-derived stromal cel ls into astrocytes. Neural Regen Res. 2011;6(10): 738-743.[20] Baer PC, Griesche N, Luttmann W, et al. Human adipose-derived mesenchymal stem cells in vitro: evaluation of an optimal expansion medium preserving stemness. Cytotherapy. 2010;12(1):96-106.[21] Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human firsttrim ester fetal blood, liver and bone marrow. Blood. 2001;98(8): 2396-2402.[22] Cai YN, Yuan XD, Ou Y, et al. Apoptosis during β-mercaptoethanol-induced differentiation of adult adipose-derived stromal cel ls into neurons. Neural Regen Res. 2011;6(10):750-755.[23] Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189(1):54-63.[24] Gu F, Gao JH, Lu F. Culture of the single cell clones of human adipose-derived stem cells and identification of their surface antigen expressions. Nan Fang Yi Ke Da Xue Xue Bao. 2008; 28(6):1067-1069. [25] Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12): 4279-4295.[26] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for de?ning multipotent mesenchymal stromal cells. The international Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.[27] Varma MJ, Breuls RG, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007; 16(1): 91-104.[28] Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7(2):269-291.[29] Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693.[30] Xiao J, Yang X, Jing W, et al. Adipogenic and osteogenic differentiation of Lin(-)CD271(+)Sca-1(+) adipose-derived stem cells. Mol Cell Biochem, 2013. [Epub ahead of print].[31] Zuk P. Adipose-Derived Stem Cells in Tissue Regeneration: A Review. ISRN Stem Cells. 2013;2013:1-35.[32] Yuan XD, Cai YN, Ou Y, et al. Adult adipose-derived stromal cells differentiate into neurons with normal electrophysiological functions. Neural Regen Res. 2011;6(34): 2681-2686.[33] 王清富,陈庄洪,蔡贤华,等.兔脂肪干细胞的多向诱导分化[J].中国组织工程研究, 2012, 16(49):9162-9167.[34] Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells: the great WAT hope.Trends Endocrinol Metab. 2012;23(6):270-277.[35] Macotela Y, Emanuelli B, Mori MA, et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61(7):1691-1699.[36] Suga H, Matsumoto D, Eto H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18(8):1201-1209.[37] Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation.Am J Physiol Cell Physiol. 2008;295(4):C1037-1044.[38] Buehrer BM, Cheatham B. Isolation and characterization of human adipose-derived stem cells for use in tissue engineering. Methods Mol Biol. 2013;1001:1-11. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [14] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||