Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (19): 3103-3109.doi: 10.3969/j.issn.2095-4344.3536
Previous Articles Next Articles
Mesenchymal stem cell transplantation in the treatment of myocardial infarction: problems, crux and new breakthrough
Sun Weixing, Zhao Yongchao, Zhao Ranzun
- Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2020-05-29Revised:2020-06-11Accepted:2020-07-23Online:2021-07-09Published:2021-01-14 -
Contact:Zhao Ranzun, MD, Professor, Doctoral supervisor, Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Sun Weixing, Associate chief physician, Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:the National Natural Science Foundation of China, No. NSFC81660049 (to ZRZ)
CLC Number:
Cite this article
Sun Weixing, Zhao Yongchao, Zhao Ranzun. Mesenchymal stem cell transplantation in the treatment of myocardial infarction: problems, crux and new breakthrough[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3103-3109.
share this article
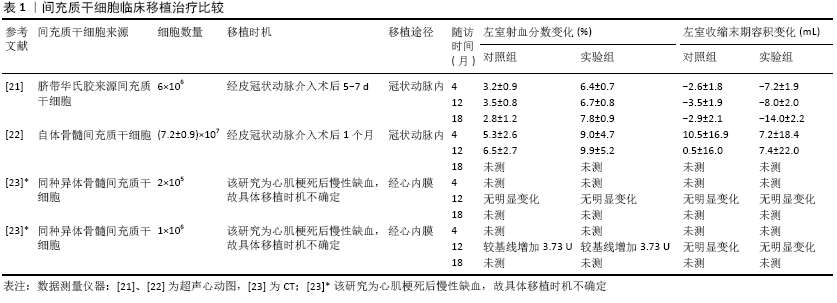
2.1 间充质干细胞的生物学特性 2.1.1 间充质干细胞增殖与多向分化潜能 间充质干细胞具有自我更新和多向分化潜能,这也是其区别于其他成体细胞的显著特征之一。间充质干细胞增殖能力强,贴壁生长快,在特定诱导下,可分化为脂肪、骨、软骨、内皮和心肌等多种细胞。诱导间充质干细胞分化为心肌细胞常用的方法包括5-氮杂胞苷、二甲基亚砜、转化生长因子β1、血管紧张素Ⅱ和机械牵拉刺激等[5-6],其中最常用的是5-氮杂胞苷[5]。近期KAKKAR等[5]发现转化生长因子β1比5-氮杂胞苷更有效和安全。 2.1.2 间充质干细胞低免疫原性和免疫调节 间充质干细胞表达CD105、CD73和CD90而不表达 CD45、CD34、CD14或 CD11b、CD79a或CD19和HLA-DR表面分子。在γ-干扰素的刺激下骨髓间充质干细胞表达少量HLA-DR(≤2%)[7-8],且在临床应用研究中发现不改变间充质干细胞的形态学、间充质表型、多向分化潜能及免疫调节能力[8]。另外,间充质干细胞不表达共刺激因子CD80、CD86和CD40等,因此间充质干细胞有低免疫原性,不能诱导免疫应答及移植排斥反应。 间充质干细胞具有较强免疫抑制作用,其在心肌梗死移植治疗中发挥重要作用。间充质干细胞对T细胞、B细胞、NK细胞、树突状细胞及巨噬细胞均有免疫抑制作用,其通过上调抗炎因子和下调促炎因子并作用于免疫细胞发挥抑制炎症反应的作用。间充质干细胞的免疫调节作用与机体的免疫系统活化状态有关,在免疫系统未过度激活时其促进炎症反应,而在过度激活时其抑制炎症反应[9]。间充质干细胞在静息和活化时表达前列腺素E2、诱导型一氧化氮合酶(鼠)、转化生长因子β、白细胞介素10、肝细胞生长因子、CD39 、CD73、半乳糖凝集素类、C‐C基序趋化因子配体2(C‐C motif chemokine ligand 2,CCL2)、肿瘤坏死因子刺激基因6和白细胞介素1受体拮抗剂。吲哚胺2,3-二加氧酶(indole-amine 2,3-dioxygenase, IDO)(人)、程序性细胞死亡配体1 和2(programmed cell death ligands 1 and 2,PD-L1和PD-L2)及补体系统相关蛋白等只在间充质干细胞活化时表 达[9-10],同时间充质干细胞通过表达趋化因子和黏附分子,如CXCR3配体、CCR5配体、细胞间黏附分子1和血管细胞黏附分子1,与免疫细胞直接接触,抑制免疫细胞的增殖及其功能[11]。近几年来,研究发现在促炎因子γ-干扰素、肿瘤坏死因子ɑ和白细胞介素1β等刺激下,间充质干细胞表达和分泌PD-L1和PD-L2并通过该配体与T细胞接触而抑制T细胞的活化和白细胞介素2的分泌以及不可逆地诱导T细胞呈低反应状态和死亡[9,12]。树突状细胞是功能最强的专职抗原呈递细胞。SHAHIR等[13]证实间充质干细胞来源的外泌体能将成熟和未成熟的树突状细胞诱导为耐受性树突状细胞,其具体机制还不清楚。耐受性树突状细胞抑制淋巴细胞活性,减少促炎因子白细胞介素6的分泌,增加抗炎因子白细胞介素10和转化生长因子β的分泌[13],从而抑制炎症反应。耐受性树突状细胞主要通过树突状细胞上的PD-L1和PD-L2等共抑制分子与T细胞上的PD-1相互作用介导免疫抑制反应[14]。人胎盘间充质干细胞抑制单核细胞向树突状细胞分化和成熟,人胎盘间充质干细胞通过与NK细胞接触并上调NK细胞抑制性受体和下调NK细胞激活受体KP30来抑制NK细胞,从而减弱树突状细胞刺激T细胞的增殖能力[10]。人脐血间充质干细胞促进M1型巨噬细胞向具有抗炎作用的M2型转换而减轻炎症反应[15]。近年来的这些研究发现越来越多的免疫细胞受间充质干细胞抑制。间充质干细胞的免疫抑制作用是多途径、复杂的过程,且其抗炎作用是明确的,因此需要不断研究去认识其调节机制,以便实施精准干预,同时可考虑结合细胞亚群研究,为筛选理想的间充质干细胞提供理论依据。 2.1.3 损伤趋化归巢 归巢是干细胞对损伤组织发挥修复作用的重要条件。组织在缺血、缺氧等损伤时释放趋化因子、黏附分子、生长因子和酶,如基质衍生因子1(stromal cell-derived factor-1,SDF-1)、粒细胞集落刺激因子、单核细胞趋化蛋白等,它们与干细胞上相应受体结合介导干细胞迁移归巢于损伤区[16-17]。目前研究证实一些信号通路参与间充质干细胞的归巢,其中最主要的是SDF-1/CXCR4信号通路。CXCR4是SDF-1的特异性受体,SDF-1在正常成人心脏中结构性表达,在动物心肌梗死模型中SDF-1表达上调[16-17]。TIAN等[18]证实SDF-1/CXCR4信号通路在间充质干细胞归巢中发挥重要作用。TAO等[19]发现HMGB1通过作用于SDF-1/CXCR4 轴促进间充质干细胞向内皮损伤处迁移。SEGERS等[20]发现间充质干细胞与心脏内皮细胞至少部分通过VCAM-1/VLA-4通路发生黏附并促进间充质干细胞归巢。然而关于间充质干细胞损伤趋化归巢的具体调控机制仍然不清楚。 2.2 间充质干细胞移植治疗心肌梗死的细胞数量、移植途径、移植时机等 越来越多的临床前研究证明间充质干细胞移植治疗心肌梗死的有效性和安全性,但对间充质干细胞临床移植治疗的有效性存在争论,移植细胞数量、细胞输送途径及细胞移植最佳时间窗问题仍不统一,见表1。 2.2.1 间充质干细胞移植治疗的有效性 对间充质干细胞移植治疗研究,首先要解决其治疗的有效性。近年来,越来越多研究证实间充质干细胞对心肌梗死移植治疗的有效 性[21-23]。LALU等[2]对23项间充质干细胞移植治疗缺血性心脏病研究(急性心肌梗死11项和缺血性心衰12项)的安全性和有效性进行了Meta分析,其结果表明间充质干细胞对缺血性心脏疾病的治疗是安全的,但其有效性有待进一步研究。在该Meta分析中,研究者根据疾病进行亚组分析后发现同种异体骨髓间充质干细胞移植治疗患者的左室射血分数显著增加,而自体骨髓间充质干细胞则不明显。在回顾分析数据之后,作者认为不能明确界定有效性的原因主要有以下几点:①每个研究使用的测量方法不统一;②这些研究中大多应用的是自体骨髓间充质干细胞;③研究对照组设置不一致;④骨髓间充质干细胞输送途径不一致;⑤治疗后评估时间不一致;⑥骨髓间充质干细胞移植治疗剂量不一致。考虑到目前的临床前研究中间充质干细胞移植治疗后左室射血分数有明显增加,以及间充质干细胞亚群的研究越来越多和备选干细胞丰富,因此有必要精心设计研究体系对间充质干细胞移植治疗急性心肌梗死的有效性深入研究。 2.2.2 细胞数量 干细胞移植数量及次数可能对治疗效果产生影响。GAO等[21]对58例患者经冠状动脉内注射6×106个脐带华氏胶来源间充质干细胞,4个月后心肌活力及左室射血分数增加,18个月后左室射血分数较安慰剂组显著增加,左心室舒张末期容积和左心室收缩末期容积较安慰剂组明显减少。KIM等[22]对14例患者经冠状动脉内注射(7.2±0.9)×107 个自体骨髓间充质干细胞,4个月后出现左室射血分数升高。FLOREA等[23]对30例缺血性心肌病伴心功能不全患者行左心室导管经心内膜注射人骨髓间充质干细胞治疗,一组给予每人2×105个间充质干细胞,另一组给予每人106个间充质干细胞,12个月后两组心脏瘢痕面积减少相似,但射血分数只在后者中升高,该研究表明细胞剂量是影响疗效的关键因素之一。上述临床研究表明不同剂量的间充质干细胞都会取得一定的治疗效果。虽然FLOREA等[23]对细胞剂量进行了分组对比,但与另外两项研究一样没有进行间充质干细胞合适剂量的研究。XU等[24]在大鼠心肌梗死第1周或第2周多次注射间充质干细胞后,发现间充质干细胞在心肌梗死部位的募集有累计效应,尤其是注射3次时。虽然该方案对患者是否有类似的效应需要验证,但为干细胞移植治疗提供了新的思路。骨髓间充质干细胞的CD146+细胞亚群通过促进心肌细胞的增殖及减少其凋亡和缺氧产生的活性氧,恢复缺血损伤的心肌细胞[25],这表明有特异功能的细胞亚群因提高了疗效可能会减少细胞移植数量。 2.2.3 移植途径 间充质干细胞移植治疗的途径主要有经静脉、冠状动脉内、心内膜心肌内注射及心外膜心肌内注射,近年来临床前研究出现了心肌贴片及联合途径干细胞输送,每种途径都有其优缺点,静脉内注射操作方便,但归巢于心肌损伤部位的间充质干细胞较少,大部分(约70%)位于肺部,其余分布于心、肾、脾脏及膀胱[26],经介入心肌内注射技术要求较高,而开胸心肌内注射对绝大多数患者也不可取。目前,研究者们倾向于经冠状动脉内途径输送间充质干细胞。KIM等[22]对26例ST段抬高前壁心肌梗死患者在经皮冠状动脉介入术后1个月,经冠状动脉内输送自体骨髓间充质干细胞进行移植治疗,随访4个月和12个月发现左心室射血分数明显增加,未出现死亡、再住院、心肌梗死、支架血栓形成、危及生命的心律失常或中风。GAO等[21]经皮冠状动脉介入术后5-7 d经冠状动脉内注射脐带华氏胶来源间充质干细胞,4个月后出现心肌活力及左室射血分数增加。但ZLABINGER等[27] 对45头心肌梗死猪进行心肌内注射(22头)和冠状动脉内注射(23头)间充质干细胞比较研究,发现冠状动脉内注射间充质干细胞因心肌组织灌注延迟和不完全恢复导致绝对心肌血流量急速下降,引发氧化应激导致归巢因子CXCR4等减少,间充质干细胞归巢受限,心肌组织中血管生成因子减少,且预后也较差。以上研究表明间充质干细胞冠状动脉内输送可能存在影响其归巢和发挥治疗功能的潜在问题,也急需临床前深入研究去证实和解决。近年来,随着组织工程技术的发展,基于细胞治疗的心肌贴片成为研究的热点。心肌贴片有利于增加梗死区的细胞输送、增殖和迁移,移植细胞均匀分布,模拟细胞外基质重构,限制梗死区的心室重构等。DONG等[28]将间充质干细胞黏附到由组织来源细胞外基质与丝蛋白以及金纳米离子构建的生物活性支架(Au-ECM/SP)上,经开胸贴到心肌梗死大鼠的心外膜上,结果发现心肌梗死面积明显缩小(89%降到65%)。PARK等[29]在大鼠心肌梗死模型中对比心肌内注射人诱导多能干细胞(human induced pluripotent stem cells,hiPSC-CMs)联合心外膜人间充质干细胞贴片(human mesenchymal stem cell-loaded patch, hMSC-PA)与心肌内注射hiPSC-CMs、人间充质干细胞和心脏组织来源细胞外基质(heart-derived decellularized extracellular matrix,hdECM)混合物对心肌梗死的效果,发现hiPSC-CMs 联合hMSC-PA治疗组纤维化面积明显减少,毛细血管及心脏功能显著增加。以上2个临床前研究有望提高干细胞移植效率。然而LUGER等[30]认为急性心肌梗死后间充质干细胞移植治疗的获益除了来自间充质干细胞植入心肌梗死局部发挥作用外,更重要的是间充质干细胞的全身性免疫抑制作用。综上,间充质干细胞移植治疗的细胞输送途径仍然值得进一步研究,以获得机体最小损伤,间充质干细胞最大利用。 2.2.4 移植时机 心肌梗死后干细胞移植时机也存在争议,通常会在心肌梗死后1周内行干细胞移植治疗。GAO等[21]在患者经皮冠状动脉介入术后5-7 d经冠状动脉内注射6×106个脐带华氏胶来源间充质干细胞,4个月后出现心肌活力及左室射血分数增加。KIM等[22]在ST段抬高前壁心肌梗死患者经皮冠状动脉介入术后1个月行冠状动脉内注射间充质干细胞治疗,左室射血分数得到改善。XU等[24]在大鼠心肌梗死后第1周、第2周或第4周内予以1次、2次或3次静脉注射阿托伐他汀预处理的骨髓间充质干细胞,并在心肌梗死后2 h通过灌胃方法强化阿托伐他汀(每天10 mg/kg)联合治疗6周,结果发现在心肌梗死后第2周,尤其是第14天注射干细胞,干细胞的募集量及生存率、心脏功能改善、梗死面积缩小等达最佳。因此他们认为心肌梗死后第2周为干细胞移植治疗的最佳时间窗,但在第4周仍然有效。 2.3 间充质干细胞治疗心肌梗死的主要机制 2.3.1 旁分泌作用 干细胞以细胞外囊泡的方式分泌生长因子、细胞因子、趋化因子及外泌体等并作用于邻近的细胞。通过调节性旁分泌作用,改变损伤组织局部微环境,诱导损伤修复[31]。MARDPOUR等[32]对间充质干细胞来源细胞外囊泡的蛋白质组学分析发现其富含938种蛋白质,包括与细胞外基质-受体相互作用、黏着斑和疾病特异性通路相关的蛋白质。在炎症和自身免疫性疾病中白细胞介素10、肝细胞生长因子、白血病抑制因子、CCL2、VEGFC和CCL20的表达导致间充质干细胞来源细胞外囊泡迁移到损伤部位,抑制炎症和促进再生。旁分泌作用是间充质干细胞发挥治疗作用的主要机制,包括促血管生成、抗凋亡、抑制炎症和调节细胞外基质动力学[31]。人绒毛间充质干细胞的外泌体具有明显促进内皮细胞增殖和迁移的能力[33],研究进一步发现血管细胞黏附分子1(vascular cell adhesion molecule-1,VCAM-1)阳性的人绒毛间充质干细胞外泌体中高表达血管生成的细胞因子,如肝细胞生长因子、白细胞介素8、白细胞介素1β、白细胞介素1α、粒细胞-巨噬细胞集落刺激因子、粒细胞集落刺激因子、CXCL1、CCL7、ANG、ANGPT2等[32],KIM等[34]在小鼠心肌梗死模型中实行胎盘羊膜间充质干细胞、诱导多能干细胞移植实验,结果发现持续的干细胞植入与心肌活力持续增加相一致,且未发现心肌再生,其潜在的机制为干细胞持续旁分泌因子。在大鼠心肌梗死模型中给予过表达组织基质金属蛋白酶抑制剂2的人脐带间充质干细胞分泌的外泌体,发现该外泌体部分通过激活AKT/Sfrp2通路改善心脏功能、促进血管形成、抑制不良重塑及抗心肌凋亡等发挥心脏保护作用[35]。 2.3.2 免疫抑制 间充质干细胞的免疫抑制作用在心肌梗死后心脏修复过程中发挥着重要作用。急性心肌梗死时过度的炎症反应会引起心肌细胞和心脏结构功能损伤,炎症反应严重影响心肌梗死后心脏修复及瘢痕形成。心肌梗死后存在持续过度炎症反应,这可能是导致心脏功能恶化的重要原因。免疫抑制可以明显改善心室不良重塑及心功能。间充质干细胞主要通过旁分泌作用和直接细胞间接触进行免疫调节。LUGER等[30]证实间充质干细胞通过减少心肌组织中的NK细胞和中性粒细胞等浸润发挥免疫抑制作用并明显改善心脏功能、不良心室重塑及缩小梗死面积。PENG等[15]在小鼠心肌梗死中证实人脐血间充质干细胞能促进M1型巨噬细胞向具有抗炎作用的M2型转换而减轻炎症反应,改善心功能。组织基质金属蛋白酶抑制剂2介导的基质金属蛋白酶的抑制是心肌梗死后不良重塑的关键决定因素。过表达组织基质金属蛋白酶抑制剂2的人脐带间充质干细胞分泌的外泌体部分通过激活AKT/Sfrp2通路减少胶原沉积,抑制心脏不良重塑,增加超氧化物歧化酶、谷胱甘肽过氧化物酶及减少丙二醛而减轻心肌梗死诱导的氧化应激反应等发挥心脏保护作用[35]。这些研究表明间充质干细胞通过抑制免疫细胞或基质金属蛋白酶减轻炎症反应和改善心室不良重塑。 2.3.3 促进血管生成 间充质干细胞主要通过分泌血管内皮生长因子、肝细胞生长因子等血管生成因子和分化为血管内皮细胞诱导血管生成。间充质干细胞通过一些通路途径分泌G蛋白偶联受体激酶结合蛋白1调节Notch/NF-κB信号通 路[36],分泌Erb-b2受体酪氨酸激酶4(Erb-B2 receptor tyrosine kinase 4,ERBB4)激活PI3K/AKT和MAPK/ERK信号通路[37]。在低氧时,PI3K/AKT通路促进间充质干细胞分化为血管内皮细胞及增加血管内皮生长因子等的分泌促进血管生成[38]。肝细胞生长因子是血管生成的关键因子,PI3K/AKT通路参与了肝细胞生长因子促血管生成的调节[39]。间充质干细胞外泌体促进血管内皮细胞增殖、迁移和成管能力[40],HMGB1通过作用于MAPK及p53两条信号通路促进间充质干细胞向内皮细胞分化[19]。一些细胞亚群有较强的血管生成能力,如DU等[33]在基质胶实验中观察到人绒毛间充质干细胞血管形成,尤其是血管细胞黏附分子1阳性人绒毛间充质干细胞,并检测到内皮细胞和平滑肌细胞;在肢体缺血模型中观察到血管细胞黏附分子1阳性人绒毛间充质干细胞产生明显的侧支循环并改善肢体缺血,具有显著血管生成能力[34], VENTURA FERREIRA等[41]发现人绒毛间充质干细胞高表达STRO-1、CD146及α-SMA等与血管生成和平滑肌生成相关分子,并促进血管生成。人脂肪来源间充质干细胞通过分泌白细胞介素6激活M2型巨噬细胞形成新生血管及组织修复[42]。间充质干细胞促进血管生成机制亦未完全清楚。 2.3.4 心肌梗死后心脏微环境对间充质干细胞移植的影响 急性心肌梗死后,心脏微环境对移植细胞疗效起着决定性作用[43],这可能是导致干细胞移植有效性差的重要原因之一。缺氧及心肌损伤引起炎性反应,分泌促炎因子,形成具有细胞毒性的炎性微环境,一方面引起移植干细胞的损伤、凋亡及抑制其增殖和分化,另一方面促进心肌细胞损伤加重[43],这些都不利于心脏组织的修复和心功能的改善。促进移植细胞损伤、死亡的机制主要有心脏组织炎症反应、缺氧、细胞与细胞接触的丧失、一些细胞毒性和(或)促凋亡因子[43]。因此,在间充质干细胞植入前改善梗死心肌微环境将有助于减轻炎性环境对间充质干细胞的抑制作用。改善心肌梗死微环境研究有4个方面[43]:①同时移植间充质干细胞和其他有用的干细胞;②使用干细胞分泌物;③使用预处理和修饰的干细胞;④在细胞治疗前使用抗炎药(如阿托伐他汀等)。作者认为还应积极改善局部缺血缺氧环境,如早期的溶栓、经皮冠状动脉介入术等。总之,在增强间充质干细胞自身抗炎能力同时,应积极干预微环境,选择合适的细胞移植时机,提高间充质干细胞的疗效。 2.3.5 分化为心肌细胞 间充质干细胞分化为心肌细胞存在争论,有的研究认为其不能分化为心肌细胞[44-46],有的研究则证实其可以分化为心肌细胞并改善心功能及减少梗死面积[6,47]。EMAMI等[6]用5-氮杂胞苷化学刺激和机械牵拉2种方法均可使间充质干细胞分化为心肌细胞,并在心肌梗死模型中移植预分化的间充质干细胞,2个月后发现相对于未预分化组,预分化组的间充质干细胞明显缩小心肌梗死面积和改善左室射血分数(脂肪来源间充质干细胞组和骨髓来源间充质干细胞组左室射血分数水平分别提高了13.4%和12.1%)。至今,成年间充质干细胞在体外可通过模拟体内条件(包括基质、细胞连接性、其他细胞类型的相互作用和电刺激)来产生功能性心肌细胞尚无共识[6]。因此间充质干细胞心肌分化潜能及其分化是否有意义仍需进一步研究。 2.4 RNA m6A甲基化修饰与间充质干细胞 近几年来,RNA的表观遗传修饰成了研究的热点,为一些疾病治疗提供新的方法。m6A是真核生物中最常见表观遗传修饰,在调节多种mRNA的稳定性、翻译中起着重要作用,并参与多种细胞过程。已有研究证实RNA m6A甲基化修饰具有调控其他干细胞增殖、分化等作用。SHEN等[3]发现去甲基化酶ALKBH5通过ALKBH5/m6A/TACC3轴调节白血病干细胞的自我更新,促进急性髓系白血病的发展。CAO等[48]发现去甲基化酶Fto通过作用于Pdgfra/Socs5-Stat3通路调节成年神经干细胞的增殖和分化。那么RNA m6A甲基化修饰又会对间充质干细胞产生哪些影响呢?迄今为止,对间充质干细胞中RNA m6A甲基化修饰研究很少,如WU等[49]发现RNA m6A甲基化酶METTL3促进骨髓间充质干细胞向成骨细胞分化。通过对间充质干细胞RNA m6A甲基化修饰来进一步改善间充质干细胞的生存率及提高其生物学功能,有望为间充质干细胞移植治疗开辟新的领域。"
| [1] 胡盛寿,高润霖,刘力生,等.《中国心血管病报告2018》概要[J].中国循环杂志,2019,34(3):209-220. [2] LALU MM, MAZZARELLO S, ZLEPNIG J, et al. Safety and Efficacy of Adult Stem Cell Therapy for Acute Myocardial Infarction and Ischemic Heart Failure (SafeCell Heart): A Systematic Review and Meta-Analysis. Stem Cells Transl Med. 2018;7(12):857-866. [3] SHEN C, SHENG Y, ZHU AC, et al. RNA Demethylase ALKBH5 Selectively Promotes Tumorigenesis and Cancer Stem Cell Self-Renewal in Acute Myeloid Leukemia. Cell Stem Cell. 2020;27(1):64-80.e9. [4] BOO SH, KIM YK. The emerging role of RNA modifications in the regulation of mRNA stability. Exp Mol Med. 2020;52(3):400-408. [5] KAKKAR A, NANDY SB, GUPTA S, et al. Adipose tissue derived mesenchymal stem cells are better respondents to TGFβ1 for in vitro generation of cardiomyocyte-like cells. Mol Cell Biochem. 2019; 460(1-2):53-66. [6] ABD EMAMI B, MAHMOUDI E, SHOKRGOZAR MA, et al. Mechanical and Chemical Predifferentiation of Mesenchymal Stem Cells Into Cardiomyocytes and Their Effectiveness on Acute Myocardial Infarction. Artif Organs. 2018;42(6):E114-E126. [7] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [8] GRAU-VORSTER M, LAITINEN A, NYSTEDT J, et al. HLA-DR expression in clinical-grade bone marrow-derived multipotent mesenchymal stromal cells: a two-site study. Stem Cell Res Ther. 2019;10(1):164. [9] JIANG W, XU J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53(1):e12712. [10] ABUMAREE MH, ABOMARAY FM, ALSHABIBI MA, et al. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta. 2017;59:87-95. [11] WANG Y, CHEN X, CAO W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009-1016. [12] DAVIES LC, HELDRING N, KADRI N, et al. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017;35(3):766-776. [13] SHAHIR M, MAHMOUD HASHEMI S, et al. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J Cell Physiol. 2020;235(10):7043-7055. [14] YOO S, HA SJ. Generation of Tolerogenic Dendritic Cells and Their Therapeutic Applications. Immune Netw. 2016;16(1):52-60. [15] PENG Y, CHEN B, ZHAO J, et al. Effect of intravenous transplantation of hUCB-MSCs on M1/M2 subtype conversion in monocyte/macrophages of AMI mice. Biomed Pharmacother. 2019;111:624-630. [16] TAO Z, TAN S, CHEN W, et al. Stem Cell Homing: a Potential Therapeutic Strategy Unproven for Treatment of Myocardial Injury. J Cardiovasc Transl Res. 2018;11(5):403-411. [17] LI X, HE XT, YIN Y, et al. Administration of signalling molecules dictates stem cell homing for in situ regeneration. J Cell Mol Med. 2017;21(12): 3162-3177. [18] TIAN XQ, YANG YJ, LI Q, et al. Combined therapy with atorvastatin and atorvastatin-pretreated mesenchymal stem cells enhances cardiac performance after acute myocardial infarction by activating SDF-1/CXCR4 axis. Am J Transl Res. 2019;11(7):4214-4231. [19] TAO X, SUN M, CHEN M, et al. HMGB1-modified mesenchymal stem cells attenuate radiation-induced vascular injury possibly via their high motility and facilitation of endothelial differentiation. Stem Cell Res Ther. 2019;10(1):92. [20] SEGERS VF, VAN RIET I, ANDRIES LJ, et al. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am J Physiol Heart Circ Physiol. 2006;290(4):H1370-1377. [21] GAO LR, CHEN Y, ZHANG NK, et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162. [22] KIM SH, CHO JH, LEE YH, et al. Improvement in Left Ventricular Function with Intracoronary Mesenchymal Stem Cell Therapy in a Patient with Anterior Wall ST-Segment Elevation Myocardial Infarction. Cardiovasc Drugs Ther. 2018;32(4):329-338. [23] FLOREA V, RIEGER AC, DIFEDE DL, et al. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ Res. 2017;121(11): 1279-1290. [24] XU J, XIONG YY, LI Q, et al. Optimization of Timing and Times for Administration of Atorvastatin-Pretreated Mesenchymal Stem Cells in a Preclinical Model of Acute Myocardial Infarction. Stem Cells Transl Med. 2019;8(10):1068-1083. [25] ZHANG B, ZHANG J, ZHU D, et al. Mesenchymal stem cells rejuvenate cardiac muscle after ischemic injury. Aging (Albany NY). 2019;11(1): 63-72. [26] ASSIS AC, CARVALHO JL, JACOBY BA, et al. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19(2):219-230. [27] ZLABINGER K, LUKOVIC D, HEMETSBERGER R, et al. Matrix Metalloproteinase-2 Impairs Homing of Intracoronary Delivered Mesenchymal Stem Cells in a Porcine Reperfused Myocardial Infarction: Comparison With Intramyocardial Cell Delivery. Front Bioeng Biotechnol. 2018;6:35. [28] DONG Y, HONG M, DAI R, et al. Engineered bioactive nanoparticles incorporated biofunctionalized ECM/silk proteins based cardiac patches combined with MSCs for the repair of myocardial infarction: In vitro and in vivo evaluations. Sci Total Environ. 2020;707:135976. [29] PARK SJ, KIM RY, PARK BW, et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 2019;10(1):3123. [30] LUGER D, LIPINSKI MJ, WESTMAN PC, et al. Intravenously Delivered Mesenchymal Stem Cells: Systemic Anti-Inflammatory Effects Improve Left Ventricular Dysfunction in Acute Myocardial Infarction and Ischemic Cardiomyopathy. Circ Res. 2017;120(10):1598-1613. [31] GAGGI G, IZZICUPO P, DI CREDICO A, et al. Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine. Int J Mol Sci. 2019; 20(7):1573. [32] MARDPOUR S, HAMIDIEH AA, TALEAHMAD S, et al. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol. 2019;234(6):8249-8258. [33] DU W, LI X, CHI Y, et al. VCAM-1+ placenta chorionic villi-derived mesenchymal stem cells display potent pro-angiogenic activity. Stem Cell Res Ther. 2016;7:49. [34] KIM PJ, MAHMOUDI M, GE X, et al. Direct evaluation of myocardial viability and stem cell engraftment demonstrates salvage of the injured myocardium. Circ Res. 2015;116(7):e40-50. [35] NI J, LIU X, YIN Y, et al. Exosomes Derived from TIMP2-Modified Human Umbilical Cord Mesenchymal Stem Cells Enhance the Repair Effect in Rat Model with Myocardial Infarction Possibly by the Akt/Sfrp2 Pathway. Oxid Med Cell Longev. 2019;2019:1958941. [36] LI L, TANG P, ZHOU Z, et al. GIT1 regulates angiogenic factor secretion in bone marrow mesenchymal stem cells via NF-κB/Notch signalling to promote angiogenesis. Cell Prolif. 2019;52(6):e12689. [37] LIANG X, DING Y, LIN F, et al. Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. FASEB J. 2019;33(3):4559-4570. [38] SHENG L, MAO X, YU Q, et al. Effect of the PI3K/AKT signaling pathway on hypoxia-induced proliferation and differentiation of bone marrow-derived mesenchymal stem cells. Exp Ther Med. 2017;13(1):55-62. [39] TU J, WAN C, ZHANG F, et al. Genetic correction of Werner syndrome gene reveals impaired pro-angiogenic function and HGF insufficiency in mesenchymal stem cells. Aging Cell. 2020;19(5):e13116. [40] ZHANG L, JIAO G, REN S, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11(1):38. [41] VENTURA FERREIRA MS, BIENERT M, Müller K, et al. Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Res Ther. 2018;9(1):28. [42] PILNY E, SMOLARCZYK R, JAROSZ-BIEJ M, et al. Human ADSC xenograft through IL-6 secretion activates M2 macrophages responsible for the repair of damaged muscle tissue. Stem Cell Res Ther. 2019;10(1):93. [43] KHODAYARI S, KHODAYARI H, AMIRI AZ, et al. Inflammatory Microenvironment of Acute Myocardial Infarction Prevents Regeneration of Heart with Stem Cells Therapy. Cell Physiol Biochem. 2019;53(5):887-909. [44] MARTIN-RENDON E, SWEENEY D, LU F, et al. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008;95(2):137-148. [45] LEE WC, SEPULVEDA JL, RUBIN JP, et al. Cardiomyogenic differentiation potential of human adipose precursor cells. Int J Cardiol. 2009;133(3): 399-401. [46] WAN SAFWANI WK, MAKPOL S, SATHAPAN S, et al. 5-Azacytidine is insufficient for cardiogenesis in human adipose-derived stem cells. J Negat Results Biomed. 2012;11:3. [47] XU H, ZHOU Q, YI Q, et al. Islet-1 synergizes with Gcn5 to promote MSC differentiation into cardiomyocytes. Sci Rep. 2020;10(1):1817. [48] CAO Y, ZHUANG Y, CHEN J, et al. Dynamic effects of Fto in regulating the proliferation and differentiation of adult neural stem cells of mice. Hum Mol Genet. 2020;29(5):727-735. [49] WU Y, XIE L, WANG M, et al. Mettl3-mediated m6A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. 2018;9(1):4772. [50] DENG R, LIU Y, HE H, et al. Haemin pre-treatment augments the cardiac protection of mesenchymal stem cells by inhibiting mitochondrial fission and improving survival. J Cell Mol Med. 2020;24(1):431-440. [51] MAO Q, LIANG XL, WU YF, et al. ILK promotes survival and self-renewal of hypoxic MSCs via the activation of lncTCF7-Wnt pathway induced by IL-6/STAT3 signaling. Gene Ther. 2019;26(5):165-176. [52] SONG X, SU L, YIN H, et al. Effects of HSYA on the proliferation and apoptosis of MSCs exposed to hypoxic and serum deprivation conditions. Exp Ther Med. 2018;15(6):5251-5260. [53] KORNICKA K, MARYCZ K, MARĘDZIAK M, et al. The effects of the DNA methyltranfserases inhibitor 5-Azacitidine on ageing, oxidative stress and DNA methylation of adipose derived stem cells. J Cell Mol Med. 2017;21(2):387-401. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [5] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [6] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [7] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [8] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [9] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [10] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [11] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [12] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [13] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [14] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [15] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||