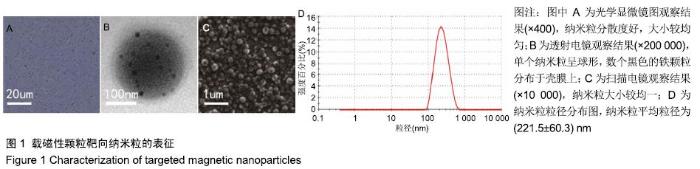
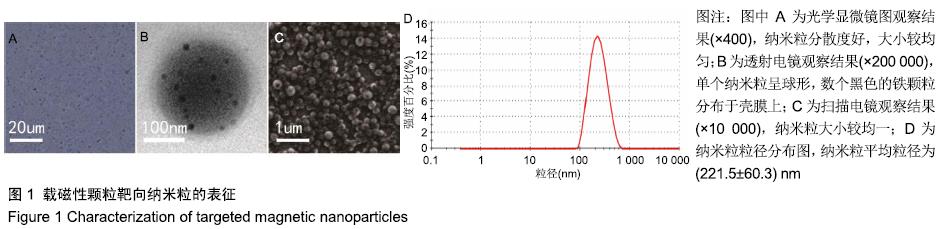
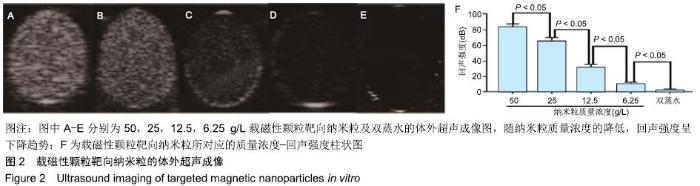
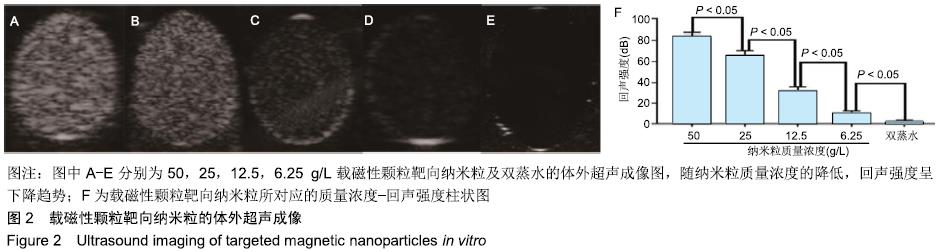
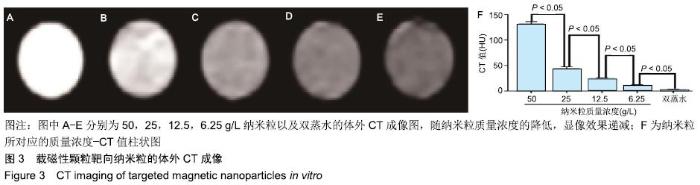
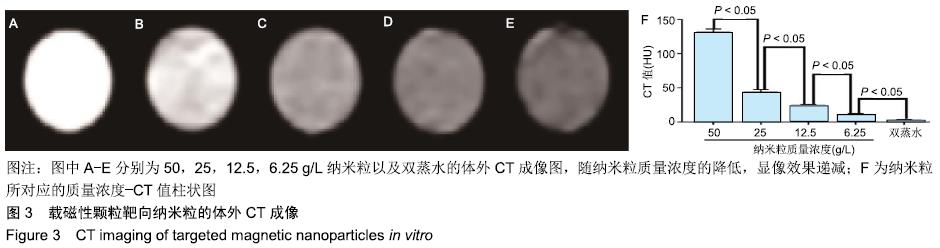
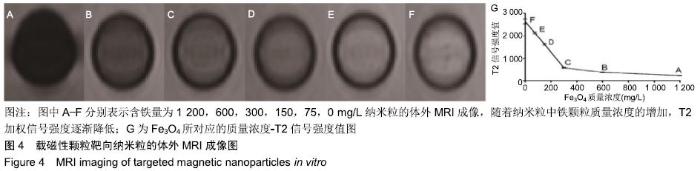
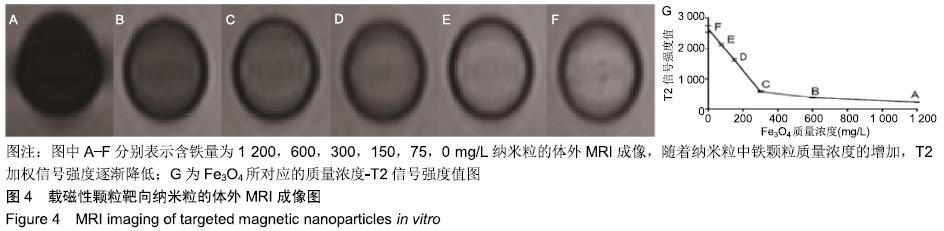
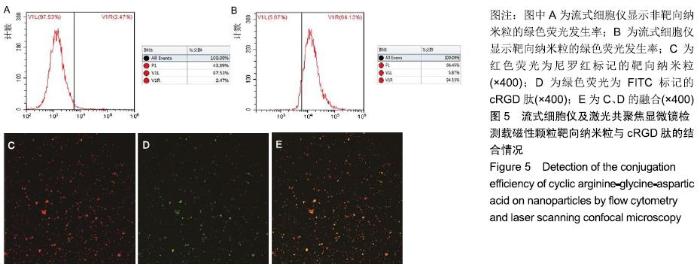
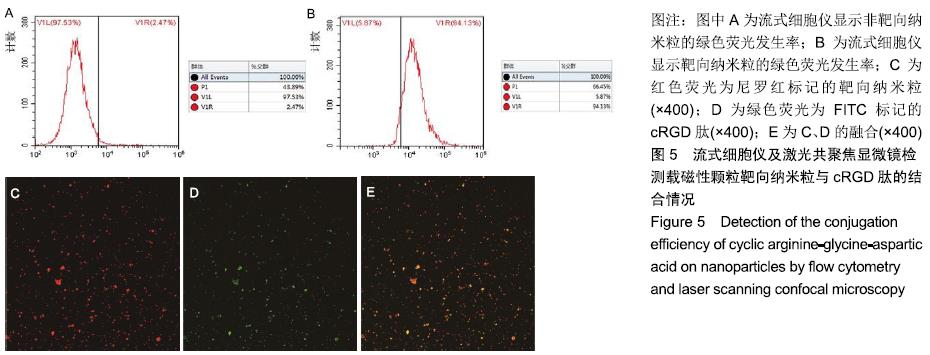
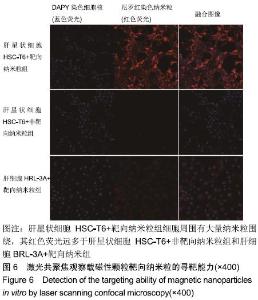
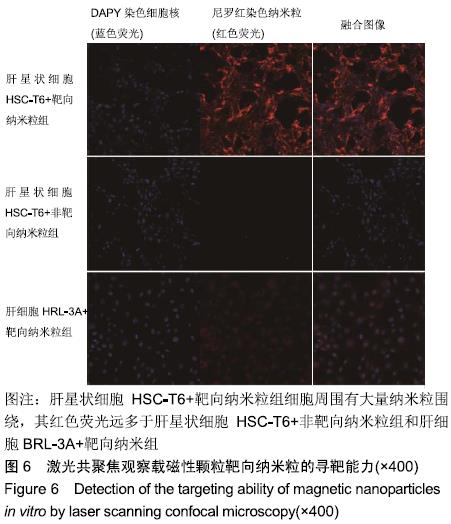
[1] POILIL SURENDRAN S, GEORGE THOMAS R, MOON MJ, et al. Nanoparticles for the treatment of liver fibrosis. Int J Nanomedicine. 2017;12:6997-7006.
[2] ALTAMIRANO-BARRERA A, BARRANCO-FRAGOSO B, MÉNDEZ-SÁNCHEZ N.[u][/u] Management strategies for liver fibrosis. Ann Hepatol.2017;16(1):48-56.
[3] MOKDAD AA, LOPEZ AD, SHAHRAZ S, et al.Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis.BMC Med. 2014;12(1):145.
[4] CAMPANA L, IREDALE JP.Regression of Liver Fibrosis. Semin Liver Dis. 2017;58(1):1-10.
[5] POVERO D, BUSLETTA C, NOVO E, et al.Liver fibrosis: A dynamic and potentially reversible process. Histol Histopathol. 2010;25(8): 1075-1091.
[6] HANSEN T, CHRISTENSEN E.Cirrhosis and liver fibrosis are potentially reversible. Ugeskr Laeger.2015;177(48):V06150527.
[7] WEISSLEDER R, ROSS BD, REHEMTULLA A, et al.Molecular Imaging: Principles and Practice.J Nucl Med.2010;52(6):1003-1003.
[8] LIMING D, XIAOJUN C, DANLI S, et al.A Laser-Activated Biocompatible Theranostic Nanoagent for Targeted Multimodal Imaging and Photothermal Therapy. Theranostics. 2017;7(18): 4410-4423.
[9] FREISE AC, WU AM.In vivo imaging with antibodies and engineered fragments.Mol Immunol.2015; 67(2):S0161589015003600.
[10] ZHOU Z, LU ZR.Molecular imaging of the tumor microenvironment. Adv Drug Deliv Rev.2016;113:24-48.
[11] PATERNOSTRO C. Cellular and molecular mechanisms in liver fibrogenesis.Archives of Biochemistry & Biophysics. Arch Biochem Biophys.2014;548(1):20-37.
[12] MALLAT A, LOTERSZTAJN S.Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis.Am J Physiol Cell Physiol. 2013;305(8): C789.
[13] ELPEK GÖ.Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update.World J Gastroenterol.2014;20(23):7260-7276.
[14] WALLACE K, BURT A, WRIGHT M.Liver fibrosis. Biochem J. 2008; 411(1):1-18.
[15] ZHANG CY, YUAN WG, HE P, et al.Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol.2016;22(48):10512-10522.
[16] HUANG Y, DENG X, LIANG J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp Cell Res.2017;352(2):420-426.
[17] SEKI E, BRENNER DA .Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015; 22(7):512-518.
[18] MALLAT A , LODDER J, TEIXEIRA-CLERC F, et al. Autophagy: A Multifaceted Partner in Liver Fibrosis. Biomed Res Int.2014;2014:869390.
[19] YANG J, HOU Y, JI G, et al.Targeted delivery of the RGD-labeled biodegradable polymersomes loaded with the hydrophilic drug oxymatrine on cultured hepatic stellate cells and liver fibrosis in rats. Eur J Pharm Sci.2014;52(1):180-190.
[20] ZHANG C, LIU H, CUI Y, et al. Molecular magnetic resonance imaging of activated hepatic stellate cells with ultrasmall superparamagnetic iron oxide targeting integrin αvβ3 for staging liver fibrosis in rat model. Int J Nanomedicine.2016;11:1097-1108.
[21] LI F, YAN H, WANG J, et al.Non-invasively differentiating extent of liver fibrosis by visualizing hepatic integrin αvβ3 expression with an MRI modality in mice. Biomaterials.2016;102:162-174.
[22] ZHANG X, GUO Q, SHI Y, et al.99m Tc-3PRGD2 scintigraphy to stage liver fibrosis and evaluate reversal after fibrotic stimulus withdrawn. Nucl Med Biol.2017;49:44-49.
[23] YU X, WU Y, LIU H, et al.Small-Animal SPECT/CT of the Progression and Recovery of Rat Liver Fibrosis by Using an Integrin αvβ3-targeting Radiotracer.Radiology.2015;279(2):502-512.
[24] PLASSARD AJ, D'HAESE PF, PALLAVARAM S, et al. Multi-Modal and Targeted Imaging Improves Automated Mid-Brain Segmentation. Proc SPIE Int Soc Opt Eng.2017;10133.pii: 101330J. doi: 10.1117/12.2254428.
[25] ZHOU J, MENG L, SUN C, et al.A "Protective Umbrella" Nanoplatform for Loading ICG and Multi-modal Imaging-guided Phototherapy. Nanomedicine.2018;14(2):289-301.
[26] LI X, SUI Z, LI X, et al.Perfluorooctylbromide nanoparticles for ultrasound imaging and drug delivery. Int J Nanomedicine. 2018;13: 3053-3067.
[27] 王志刚.多功能超声分子探针显像与增效高强度聚焦超声治疗[J].临床超声医学杂志,2017,19(9):577-579.
[28] NIU C, WANG Z, LU G, et al.Doxorubicin loaded superparamagnetic PLGA-iron oxide multifunctional microbubbles for dual-mode US/MR imaging and therapy of metastasis in lymph nodes. Biomaterials. 2013;34(9):2307-2317.
[29] SUN Y, ZHENG Y, RAN H, et al.Superparamagnetic PLGA-iron oxide microcapsules for dual-modality US/MR imaging and high intensity focused US breast cancer ablation.Biomaterials. 2012;33(24): 5854-5864.
[30] RODRIGUES M, DE LA TORRE BG, ANDREU D, et al. Kinetic uptake profiles of cell penetrating peptides in lymphocytes and monocytes. Biochim Biophys Acta.2013;1830(10):4554-4563.
[31] UUSNA J, LANGEL K, LANGEL Ü.Toxicity, Immunogenicity, Uptake, and Kinetics Methods for CPPs. Methods Mol Biol.2015;1324:133-148.
|