Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (13): 1996-2004.doi: 10.3969/j.issn.2095-4344.2048
Previous Articles Next Articles
Therapeutic effect of human adipose stem cells derived exosomes on carbon tetrachloride induced liver fibrosis in rats
Li Hongchao1, Wang Xi1, Li Li2, Li Zhenyu3, Zang Zusheng3, Zhou Heng3, Wang Xiaojin3, Chen Chengwei3, Cheng Mingliang1, Wu Jun1, #br# Jin Yinpeng2, Fu Qingchun2
- 1Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, China; 3Shanghai Liver Diseases Research Center, the 905 Hospital of PLA Navy, Shanghai 200235, China
-
Received:2019-03-13Revised:2019-03-18Accepted:2019-04-30Online:2020-05-08Published:2020-03-07 -
Contact:Fu Qingchun, Master, Chief physician, Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, China Wu Jun, Master, Chief physician, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China Jin Yinpeng, Master, Attending physician, Shanghai Public Health Clinical Center, Fudan University, Shanghai 201508, China -
About author:Li Hongchao, Master, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China Wang Xi, Master, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China Both Li Hongchao and Wang Xi contributed equally to this paper. -
Supported by:Nanjing Military Region Medical Innovation Major Project, No. 14ZX01; China Hepatitis Prevention Foundation - Tianqing Liver Disease Research Foundation, No. TQGB20150104
CLC Number:
Cite this article
Li Hongchao, Wang Xi, Li Li, Li Zhenyu, Zang Zusheng, Zhou Heng, Wang Xiaojin, Chen Chengwei, Cheng Mingliang, Wu Jun, Jin Yinpeng, Fu Qingchun. Therapeutic effect of human adipose stem cells derived exosomes on carbon tetrachloride induced liver fibrosis in rats [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(13): 1996-2004.
share this article
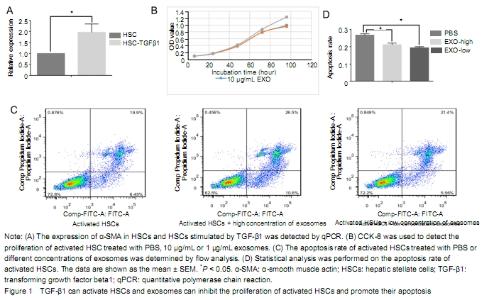
Separation and identification of hASCs and exosomes Under the inverted microscope, hASCs were fibrous, uniform in size and grew in an eddy-shaped manner. Flow cytometry showed that CD molecules on the surface of hASCs were positive for CD90, CD44, CD105 and CD73, but negative for CD34, CD19 and CD45. After cultured in lipid-induced medium, hASCs were stained with oil red O. Red lipid droplets were observed under optical fiber microscopy. After the differentiation of hASCs was induced by osteogenic induction medium, a large number of red nodules could be seen in intracellular calcium nodules stained with Alizarin red. Under scanning electron microscopy, exosomes were observed as uniform round cup shapes with vesicular structures between 30 and 150 nm in size. The diameter of exosomes was 30-150 nm by Nanosight granulometer. The results of antibody microarray showed that FLOT1 (flotillin 1), ICAM1 (intercellular adhesion molecule 1), ALIX, CD81, CD63, EpCAM (epithelial cell adhesion molecule), ANXA5 (annexin A5), TSG101 (tumor susceptibility gene 101) were all positive, while GM130 (cis-Golgi matrix protein) was negative. Histogram statistical analysis showed that the gray values of FLOT1, ICAM, ALIX, CD81, CD63, EpCAM, ANXA5 and TSG101 were all higher than those of the blank group (data not shown). Effect of exosomes on proliferation and apoptosis of activated HSCs The expression level of α-SMA in HSCs stimulated by TGF-β1 was significantly higher than that of HSCs. It indicates that TGF-β1 could activate HSCs (Figure 1A). Compared with the PBS group, the proliferation of activated HSCs was inhibited by exosome, especially at 96 hours. However, there was no significant difference between the 10 μg/L and 1 μg/L exosome treatment groups. It indicates that exosome could inhibit the proliferation of activated HSC (Figure 1B). As shown in Figure 3C, the late apoptosis percentage of activated HSCs in the high or low concentration exosomes groups and control group was 26.5%, 21.4%, and 19.9% respectively, while the early apoptosis percentage was 10.6%, 5.56%, and 6.45% respectively. The early and late apoptosis rate of high concentration exosome treatment group was higher than that of the PBS group. The late apoptosis rate in the low concentration exosome treatment group was higher than that in the PBS group, while the early apoptosis rate in the low concentration exosome treatment group was lower than that of the PBS group (Figure 1C). Compared with PBS and low-concentration exosome treatment groups, the treatment of activated HSCs with high concentration exosomes can significantly promote later apoptosis. It indicates that the apoptosis of activated HSC was significantly promoted when the exosome concentration reached a certain level (Figure 1D). "
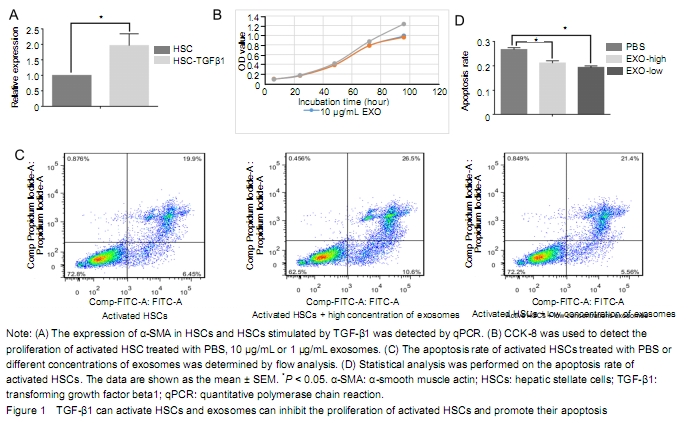
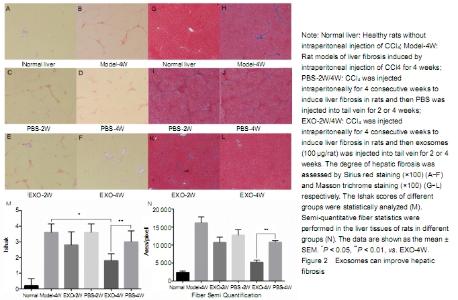
Quantitative analysis of experimental animals All rats were involved in the result analysis. Effect of exosomes on liver fibrosis CCl4 was injected intraperitoneally for 4 weeks to induce liver fibrosis. Sirius red and Masson trichrome staining of liver tissue showed moderate to severe liver fibrosis. Continuous tail venous injection of exosomes alleviated liver fibrosis, with the best effect at 4 weeks compared with the other groups (Figure 2A-L). After liver staining, Ishak was scored and compared statistically (Figure 2M). After intraperitoneal injection of CCl4 in rats, Ishak score reached 3-4, indicating moderate and severe fibrosis has formed. Compared with the rat models of liver fibrosis without PBS or exosome therapy, the Ishak scores of PBS treatment at 2 or 4 weeks were insignificantly different, indicating that the establishment of liver fibrosis model in rats is relatively stable. After 4 weeks of treatment with exosomes, liver fibrosis was significantly reduced. Semi-quantitative fiber statistics showed that exosomes significantly reduced the degree of liver fibrosis after 4 weeks of treatment (Figure 2N). No animals died during the experiment. "
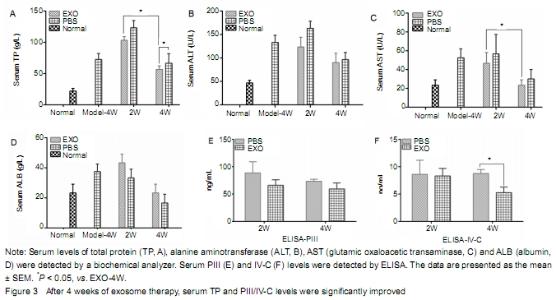
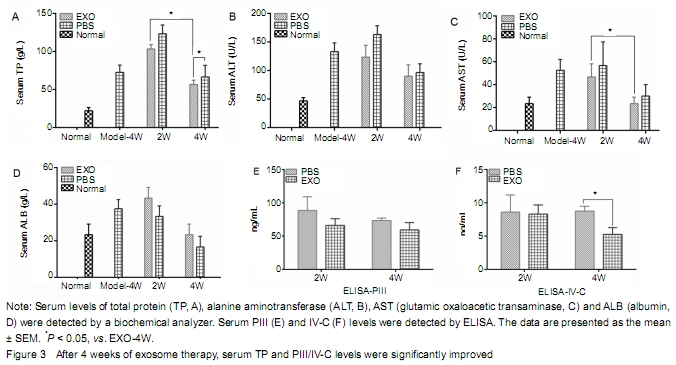
Effects of exosomes on serum liver function, type PIII and IV-C collagen in rats with liver fibrosis Compared with the exosome treatment group, serum ALT and AST levels were slightly higher, while serum TP and ALB levels were lower in the PBS group, indicating that exosome can improve the liver function. Serum TP level in the EXO-4W group was significantly higher than that in the PBS-4W group (P < 0.05). However, there was no significant difference in serum albumin level between exosomes and PBS groups (Figure 3A-D). It suggests that the effect of exosomes on the recovery of hepatic enzymes is not obvious in rats with liver fibrosis. Serum PIII and IV-C collagen levels in the exosome treatment group were lower than those in the PBS group, and there was statistical significance between EXO-4W and PBS-4W (P < 0.05) (Figure 3E-F). The results showed that exosomes decreased the expression of serum PIII/IV-C and alleviated liver fibrosis. "
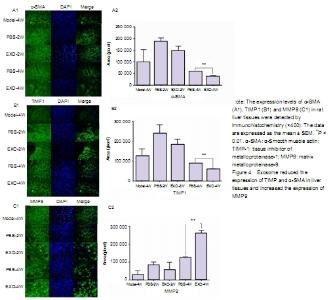
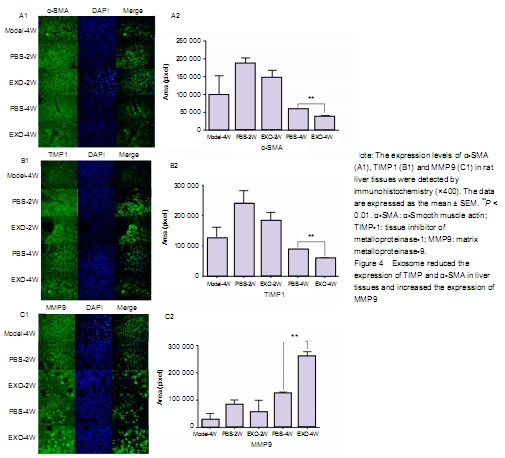
Effect of exosomes on the ECM protein expression in rats with liver fibrosis Immunohistochemical staining showed that the expression of α-SMA and TIMP1 was high in liver tissue of rats with liver fibrosis, while the expression of MMP9 was low. With the prolongation of exosome therapy time, the expression of α-SMA and TIMP1 in liver tissue decreased gradually, and the expression of MMP9 in ECM increased gradually. There was significant difference in protein expression in ECM between PBS-4W and EXO-4W. It indicates that exosomes could inhibit activated HSCs to produce α-SMA and TIMP1, and promote HSCs to produce MMP9. "
| [1] LI T, YAN Y, WANG B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6):845-854. [2] MOTAVAF M, PAKRAVAN K, BABASHAH S, et al. Therapeutic application of mesenchymal stem cell-derived exosomes: a promising cell-free therapeutic strategy in regenerative medicine. Cell Mol Biol (Noisy-le-grand). 2016;62(7):74-79. [3] SATO M, SUZUKI S, SENOO H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28(2):105-112. [4] CHEN G, JIN Y, SHI X, et al. Adipose-derived stem cell-based treatment for acute liver failure. Stem Cell Res Ther. 2015;6:40. [5] GRIFFIN MD, RYAN AE, ALAGESAN S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91(1):40-51. [6] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8(4):315-317. [7] PITTENGER MF, MACKAY AM, BECK SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284(5411):143-147. [8] TIMMERS L, LIM SK, ARSLAN F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1(2):129-137. [9] VICKERS KC, REMALEY AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23(2):91-97. [10] KOUREMBANAS S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77: 13-27. [11] TÖGEL F, VALERIUS MT, FREEDMAN BS, et al. Repair after nephron ablation reveals limitations of neonatal neonephrogenesis. JCI Insight. 2017;2(2):e88848. [12] KINNAIRD T, STABILE E, BURNETT MS, et al. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95(4):354-363. [13] NAKAGAMI H, MAEDA K, MORISHITA R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25(12):2542-2547. [14] SHI R, JIN Y, CAO C, et al. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016;7(1):155. [15] ASLAM M, BAVEJA R, LIANG OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180(11):1122-1130. [16] CHENG K, RAI P, PLAGOV A, et al. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol. 2013;94(3):466-473. [17] KIM YS, AHN JS, KIM S, et al. The potential theragnostic (diagnostic+therapeutic) application of exosomes in diverse biomedical fields. Korean J Physiol Pharmacol. 2018;22(2):113-125. [18] WANG X, ZHANG H, BAI M, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26(3):774-783. [19] JIN Y, WANG J, LI H, et al. Extracellular vesicles secreted by human adipose-derived stem cells (hASCs) improve survival rate of rats with acute liver failure by releasing lncRNA H19. EBioMedicine. 2018;34:231-242. [20] NI J, LI H, ZHOU Y, et al. Therapeutic potential of human adipose-derived stem cell exosomes in stress urinary incontinence - an in vitro and in vivo study. Cell Physiol Biochem. 2018;48(4):1710-1722. [21] LI R, SONG J, WU W, et al. Puerarin exerts the protective effect against chemical induced dysmetabolism in rats. Gene. 2016;595(2):168-174. [22] WANG JH, CHOI MK, SHIN JW, et al. Antifibrotic effects of Artemisia capillaris and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J Ethnopharmacol. 2012; 140(1):179-185. [23] VERLOH N, PROBST U, UTPATEL K, et al. Influence of hepatic fibrosis and inflammation: Correlation between histopathological changes and Gd-EOB-DTPA-enhanced MR imaging. PLoS One. 2019;14(5):e0215752. [24] NAGAKI M, SUGIYAMA A, NAIKI T, et al. Control of cyclins, cyclin-dependent kinase inhibitors, p21 and p27, and cell cycle progression in rat hepatocytes by extracellular matrix. J Hepatol. 2000;32(3):488-496. [25] ASEF A, MORTAZ E, JAMAATI H, et al. Immunologic role of extracellular vesicles and exosomes in the pathogenesis of cystic fibrosis. Tanaffos. 2018;17(2):66-72. [26] YONG-HUA Z, XIAN-SHI T, LI-JUAN S, et al. Advances in the regulatory roles of exosomes on pathological process of schistosomiasis hepatic fibrosis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(5):596-600. [27] LAI P, CHEN X, GUO L, et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol Oncol. 2018;11(1):135. [28] JIANG W, TAN Y, CAI M, et al. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int. 2018;2018:6079642. [29] NIU WH, ZHANG JJ, ZHU ZY. Research advances in the role of mesenchymal stem cells and their exosomes in treatment of liver diseases. Zhonghua Gan Zang Bing Za Zhi. 2017;25(10):793-796. [30] LOU G, CHEN Z, ZHENG M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6):e346. [31] CHEN L, CHARRIER A, ZHOU Y, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59(3):1118-1129. [32] SEO W, EUN HS, KIM SY, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology. 2016;64(2):616-631. [33] NING L, ZHU B, GAO T. Gold nanoparticles: promising agent to improve the diagnosis and therapy of cancer. Curr Drug Metab. 2017;18(11):1055-1067. [34] GNECCHI M, DANIELI P, MALPASSO G, et al. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol. 2016;1416:123-146. [35] GNECCHI M, HE H, LIANG OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367-368. [36] PHINNEY DG, DI GIUSEPPE M, NJAH J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [37] LI HC, JIN YP, WANG X, et al. Safety of freeze-dried powder of human adipose-derived stem cells and its exosomes. Zhongguo Zuzhi Gongcheng yanjiu. 2018;22(29):4593-4600. [38] WEN D, PENG Y, LIU D, et al. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J Control Release. 2016;238:166-175. [39] ARSLAN F, LAI RC, SMEETS MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301-312. [40] FIORE EJ, MAZZOLINI G, AQUINO JB. Mesenchymal stem/stromal cells in liver fibrosis: recent findings, old/new caveats and future perspectives. Stem Cell Rev. 2015;11(4):586-597. [41] HUANG B, CHENG X, WANG H, et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 2016;14:45. [42] LI J, GHAZWANI M, ZHANG Y, et al. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J Hepatol. 2013;58(3):522-528. [43] CHUN-YAN L, ZI-YI Z, TIAN-LIN Y, et al. Liquid biopsy biomarkers of renal interstitial fibrosis based on urinary exosome. Exp Mol Pathol. 2018;105(2):223-228. [44] QU Y, ZHANG Q, CAI X, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017; 21(10):2491-2502. [45] OISHI Y, MANABE I. Adipose stem cell system. Clin Calcium. 2017; 27(6):795-801. [46] WANG JY. The study on diagnostic value of co-detection of serum IV-C HA and LN in liver fibrosis patients. Yixue Lilun yu Shjian. 2001;14(2):106-108. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Chen Jiming, Wu Xiaojing, Liu Tianfeng, Chen Haicong, Huang Chengshuo. Effects of silymarin on liver injury and bone metabolism induced by carbon tetrachloride in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1224-1228. |
| [3] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [4] | Hao Xiaona, Zhang Yingjie, Li Yuyun, Xu Tao. Bone marrow mesenchymal stem cells overexpressing prolyl oligopeptidase on the repair of liver fibrosis in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3988-3993. |
| [5] | Chen Ziyang, Pu Rui, Deng Shuang, Yuan Lingyan. Regulatory effect of exosomes on exercise-mediated insulin resistance diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4089-4094. |
| [6] | Gao Kun, Chen Dayu, Zhang Yong, Liu Weidong, Sun Shufen, Lai Wenqiang, Ma Dujun, Wu Yihong, Lin Zhanpeng, Jiang Yinglu, Yu Weiji. Achyranthes bidentata alcohol extract inhibits extracellular matrix degradation of the cartilage by regulating synovial fibroblast exosomes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3636-3640. |
| [7] | Wang Kang, Zhi Xiaodong, Wang Wei. Effect of stem cell derived exosomes on repairing peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3083-3089. |
| [8] | Zhao Shuangdan, Zheng Jiahua, Qi Wenbo, Huang Xianghua. Role and mechanism of exosomes derived from mesenchymal stem cells in reproductive system diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3097-3102. |
| [9] | Liu Feng, Zhang Yu, Wang Yanli, Luo Wei, Han Chaoshan, Li Yangxin. Application of temperature-sensitive chitosan hydrogel encapsulated exosomes in ischemic diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2479-2487. |
| [10] | Cai Yuan, Deng Chengliang. Therapeutic application of adipose stem cell-free liquid extracts: skin aging, wound healing, scar recovery and nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2097-2102. |
| [11] | Liu Tao, Zhang Nini, Huang Guilin . Relationship between extracellular vesicles and radiation-induced tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2121-2126. |
| [12] | Hu Sheng, Yuan Haiyan, Hu Meng, Jin Shanhu. Effects of moderate treadmill exercise on alpha-smooth muscle actin and type IV collagen in the liver of type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(11): 1723-1727. |
| [13] |
Xie Fei, Li Yanle, Lin Xinxiao, Hu Haiwei, Sang Zhicheng, Sun Yongsheng, Jiang Kewei, Cheng Ying, Wen Guannan, Wen Jianmin, Sun Weidong.
Mechanism underlying mechanical stress regulating fibroblasts-derived
exosomes at the osteotomized end following hallux valgus correction |
| [14] | Li Xuan, Lu Min, Li Mingxing, Ao Meng, Tang Linmei, Zeng Zhen, Hu Jingwei, Huang Zhiqiang, Xuan Jiqing. In vitro multi-modal imaging of magnetic targeted nanoparticles and their targeting effect on hepatic stellate cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 566-571. |
| [15] | Yuan Yiming, Wang Yan, Chen Chengcheng, Zhao Mingyue, Pei Fei. Efficacy of exosomes in peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 5079-5084. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||