Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (26): 4192-4198.doi: 10.3969/j.issn.2095-4344.2017.26.016
Previous Articles Next Articles
Application of adipose-derived mesenchymal stem cells/sustained-release rifampin-loaded microsphere complex in an animal model of spinal tuberculosis
- Department of Tuberculosis of Wuhan Medical Treatment Center, Wuhan 430023, Hubei Province, China
-
Received:2017-04-06Online:2017-09-18Published:2017-09-28 -
Contact:Liu Wei, Department of Tuberculosis of Wuhan Medical Treatment Center, Wuhan 430023, Hubei Province, China -
About author:Huang Zheng-hui, Attending physician, Department of Tuberculosis of Wuhan Medical Treatment Center, Wuhan 430023, Hubei Province, China -
Supported by:the National Natural Science Foundation of China, No. 30973087; the Natural Science Foundation of Hubei Province, No. 2013CFB513
CLC Number:
Cite this article
Huang Zheng-hui, Liu Wei, Wang Jing-li. Application of adipose-derived mesenchymal stem cells/sustained-release rifampin-loaded microsphere complex in an animal model of spinal tuberculosis[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(26): 4192-4198.
share this article
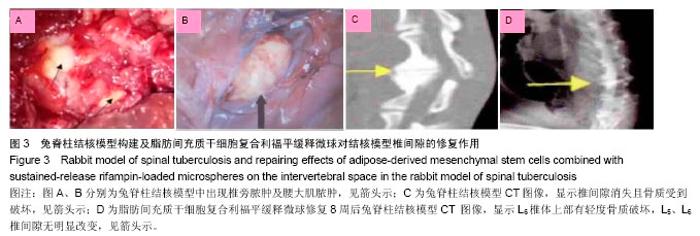
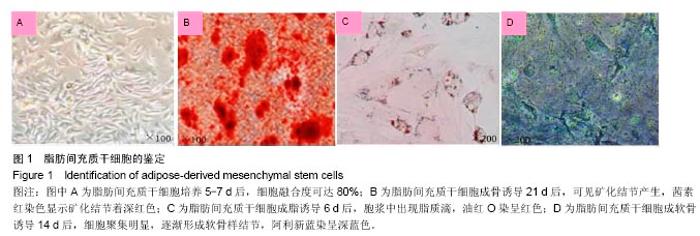
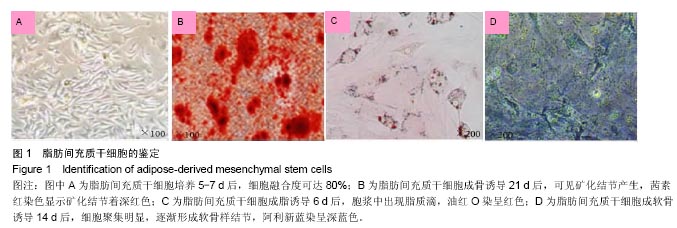
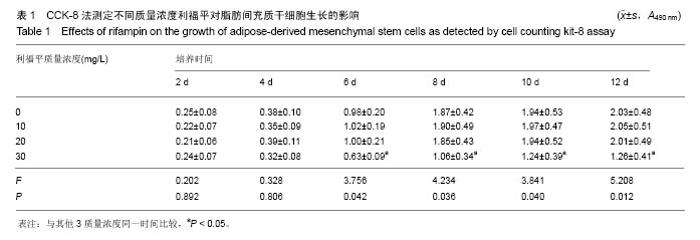
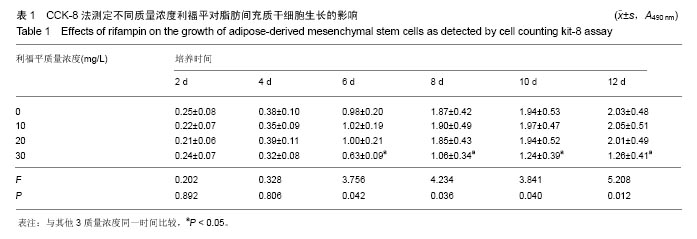
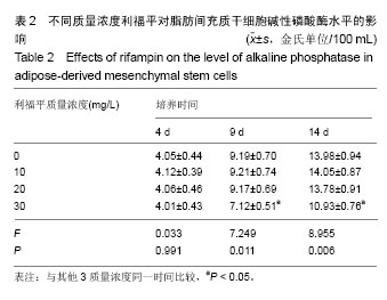
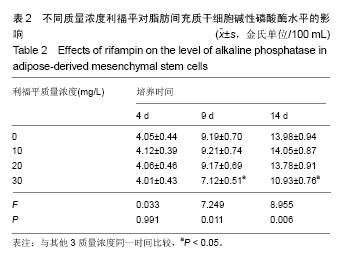
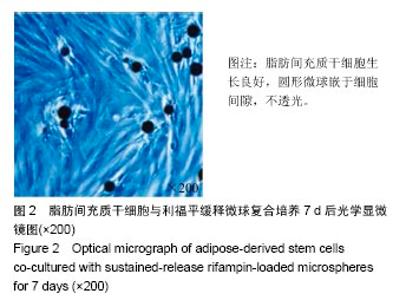
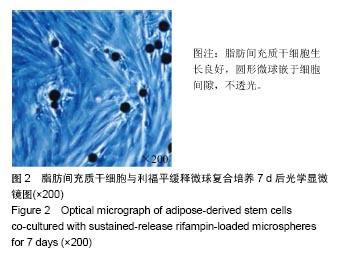
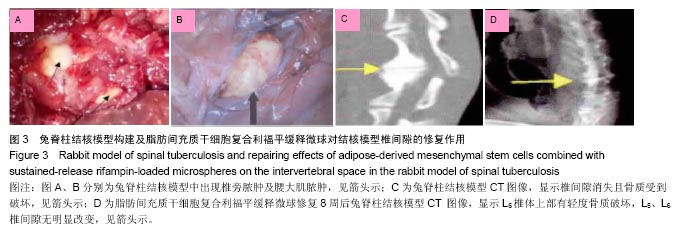
2.4 脂肪间充质干细胞复合利福平缓释微球在兔脊柱结核模型中的作用初探 兔脊柱结核模型术后2个月出现结核病灶,如椎旁脓肿(图3A)及腰大肌脓肿(图3B),L5-L6椎间盘受累,椎间隙消失且骨质受到破坏(图3C)。 给药8周后检查结果显示,对照组兔模型L5、L6椎体均有明显骨质破坏,形成炎性肉芽组织,椎间隙变窄,2只出现明显后凸畸形,5只兔腰大肌肿胀,腰大肌内可见低密度暗区,腰大肌内有1-5 cm的脓肿形成,脓液为淡黄色较浓稠,无明显臭味。利福平组兔模型中5只兔L5和L6椎体有中度骨质破坏,2只兔腰大肌肿胀。干细胞组兔模型中2只兔L5和L6椎体有中度骨质破坏,3只兔L6椎体上部有轻度骨质破坏,3只兔腰大肌肿胀。实验组中4只兔L6椎体上部有轻度骨质破坏,L5、L6椎间隙无明显改变(图3D),无腰大肌肿胀发生。 此外,4组兔肝、肺、脾组织未见明显异常,未见结核结节。 "
| [1]陶亢,张峥,呼西旦,等.兔脊柱结核模型建立的实验研究[J].新疆医科大学学报,2015, 38(8):937-940.[2]Kumar K.Spinal tuberculosis, natural history of disease, classifications and principles of management with historical perspective.Eur J Orthop Surg Traumatol.2016; 26(6): 551-558.[3]费正奇,胡蕴玉.抗骨结核缓释药物: 载体材料的选择[J].中国组织工程研究,2015,19(21): 3387-3391.[4]Kaur M,Garg T,Narang RK.A review of emerging trends in the treatment of tuberculosis.Artif Cells Nanomed Biotechnol. 2016;44(2):478-484.[5]赵宇,杨民.脂肪间充质干细胞向成骨细胞分化的研究进展[J].局解手术学杂志,2016, 25(9):694-696.[6]Wang N,Wang F,Gao Y,et al.Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis.J Pharmacol Sci. 2016; 132(3):192-200.[7]王爱欣.利福平与利福喷汀抗结核治疗效果对比分析[J].国外医药:抗生素分册,2015, 36(4):179-180.[8]张峥,姜涛,李海建,等.利福喷丁聚乳酸缓释微球对兔脊柱结核局部治疗作用的药效评价[J].新疆医科大学学报, 2015,38(8): 945-948.[9]周永泰,韩鹏,廖从辉.脊柱结核手术治疗结合术后化疗的临床效果[J].临床骨科杂志,2016, 19(6):698.[10]蒋之,屈满英,万轲.耐药脊柱结核的临床分析及疗效研究[J].中国现代医学杂志,2016, 26(11):132-136.[11]占道禄,林明侠,沈宁江,等.脊柱结核微创治疗研究的现状与进展[J].海南医学,2014, 25(2):230-232.[12]肖骁,王锡阳,罗承科.脊柱结核的外科治疗进展[J].现代实用医学, 2016,28(6): 703-704.[13]施建党,杨宗强.局部药物缓释材料在脊柱结核骨缺损修复中的应用[J].中国组织工程研究,2015,19(53):8643-8643.[14]梁求真,折胜利,宋兴华,等.体外构建抗结核性骨组织工程复合体的实验研究[J].中国矫形外科杂志,2014,22(19):1789-1796.[15]孙效虎,袁景,张宇,等.功能型骨组织工程支架修复结核性骨缺损[J].中国组织工程研究,2016,20(30):4539-4546.[16]郭欢,邵安良,程祥,等.生物人工肝用海藻酸钠/壳聚糖微囊的安全性和生物功能评价[J]. 药物分析杂志,2016,36(1):36-45.[17]李凤红,张欣蕊,相丽英,等.海藻酸钠/壳聚糖载药纳米微球的研究进展[J].化工新型材料, 2016,44(1):31-33.[18]汪程,汪令伟,史晓宇,等.海藻酸钠壳聚糖微球作为药物载体的研究进展[J].现代生物医学进展,2014,14(16): 3174-3176.[19]魏红领,刘韶瑞,陆宁.雷帕霉素-壳聚糖-海藻酸钙缓释微球抑制青光眼滤过术区瘢痕增殖的实验研究[J].眼科新进展, 2016, 36(2):110-115.[20]赛佳明,陈东亮,江晓路. BMP2-海藻酸钠-壳聚糖微球对骨折愈合影响[J].青岛大学医学院学报,2016,52(1):68-70.[21]李德超,何梦乔,李慕勤,等.纯钛微弧氧化-壳聚糖-海藻酸钠涂层的细胞相容性研究[J].口腔医学研究,2016,32(6):559-563.[22]刘苗,赵雯,李德超,等.纯钛超声微弧氧化-壳聚糖/海藻酸钠自组装种植体的动物实验研究[J].口腔医学研究, 2016,32(7): 667-671.[23]岳学锋,张俊,施建党,等.复合 HRZ/PLGA 缓释抗结核药涂层材料在兔脊柱结核病灶释药研究[J].中国脊柱脊髓杂志, 2016, 26(6):537-544.[24]Huang D,Li D,Wang T,et al.Isoniazid conjugated poly (lactide-co-glycolide): Long-term controlled drug release and tissue regeneration for bone tuberculosis therapy. Biomaterials.2015;52:417-425. [25]Shaarani S,Hamid SS,Mohd Kaus NH.The Influence of Pluronic F68 and F127 Nanocarrier on Physicochemical Properties, <i>In vitro</i> Release, and Antiproliferative Activity of Thymoquinone Drug.Pharmacognosy Res. 2017; 9(1):12-20. [26]Wang L,Yang Q,Chen Y,et al.A reformative shear precipitation procedure for the fabrication of vancomycin-loaded poly(lactide-co-glycolide) microspheres.J Biomater Appl.2017; 31(7):995-1009. [27]Abulateefeh SR,Alkawareek MY,Abdullah FR,et al. Preparation of Aqueous Core-Poly(d,l-Lactide-co-Glycolide) Shell Microcapsules With Mononuclear Cores by Internal Phase Separation: Optimization of Formulation Parameters.J Pharm Sci.2017.pii: S0022-3549(16)41953-2.doi: 10.1016/j.xphs.2016.12.027. [Epub ahead of print][28]Chen X,Chen J,Li B,et al.PLGA-PEG-PLGA triblock copolymeric micelles as oral drug delivery system: In vitro drug release and in vivo pharmacokinetics assessment.J Colloid Interface Sci.2017;490:542-552. [29]Ignjatovi? NL,Penov-Gaši KM,Wu VM,et al.Selective anticancer activity of hydroxyapatite/chitosan-poly(d,l)- lactide-co-glycolide particles loaded with an androstane-based cancer inhibitor.Colloids Surf B Biointerfaces.2016;148:629-639. [30]Alibolandi M,Abnous K,Hadizadeh F,et al.Dextran-poly lactide-co-glycolide polymersomes decorated with folate-antennae for targeted delivery of docetaxel to breast adenocarcinima in vitro and in vivo.J Control Release.2016; 241:45-56. [31]Reinbold J,Hierlemann T,Hinkel H,et al.Development and in vitro characterization of poly(lactide-<i>co</i>-glycolide) microspheres loaded with an antibacterial natural drug for the treatment of long-term bacterial infections.Drug Des Devel Ther. 2016;10:2823-2832.[32]Rumian ?,Tiainen H,Cibor U,et al.Ceramic scaffolds enriched with gentamicin loaded poly(lactide-co-glycolide) microparticles for prevention and treatment of bone tissue infections.Mater Sci Eng C Mater Biol Appl.2016;69:856-864. [33]Chu Z,Li X,Li Y,et al.Effects of different fluid shear stress patterns on the in vitro degradation of poly(lactide-co-glycolide) acid membranes.J Biomed Mater Res A.2017;105(1):23-30. [34]Bohr A,Water JJ,Wang Y,et al.Potential of surface-eroding poly(ethylene carbonate) for drug delivery to macrophages.Int J Pharm.2016;511(2):814-820. [35]Costa MP,Feitosa AC,Oliveira FC,et al.Controlled Release of Nor-β-lapachone by PLGA Microparticles: A Strategy for Improving Cytotoxicity against Prostate Cancer Cells.Molecules.2016;21(7).pii:E873.doi:10.3390/molecules21070873.[36]Sun D,Xue A,Zhang B,et al.Enhanced oral bioavailability of acetylpuerarin by poly(lactide-co-glycolide) nanoparticles optimized using uniform design combined with response surface methodology.Drug Des Devel Ther. 2016;10: 2029-2039. [37]Kavas A,Keskin D,Altunba? K,et al.Raloxifene-/ raloxifene-poly(ethylene glycol) conjugate-loaded microspheres: A novel strategy for drug delivery to bone forming cells.Int J Pharm. 2016;510(1):168-183.[38]席焱海,赵鹏,邓翠君,等.阶梯缓释抗结核纳米人工骨复合体成骨性能的实验研究[J].生物骨科材料与临床研究, 2014,11(3): 10-13,19.[39]Kim Y,Lee SH,Kang B,et al.Comparison of Osteogenesis between Adipose-Derived Mesenchymal Stem Cells and Their Sheets on Poly--Caprolactone/-Tricalcium Phosphate Composite Scaffolds in Canine Bone Defects.Stem Cells Int.2016;2016:8414715.[40]王瀚,马敏,尹峰.脂肪干细胞治疗骨关节炎的研究进展及应用前景[J].中华关节外科杂志(电子版),2016,10(3):337-340.[41]美丽,刘少英,刘福艳,等.脂肪组织来源干细胞治疗骨关节炎研究进展[J].国际骨科学杂志,2016,37(2):102-105.[42]孙玺淳,巩凡,牛东生,等.脂肪间充质干细胞结合透明质酸钠对兔骨性关节炎治疗的实验研究[J].中国矫形外科杂志, 2013, 21(19):1991-1997. |
| [1] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [2] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [3] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [4] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [5] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [6] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [7] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [8] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [9] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [10] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [11] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [12] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [13] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [14] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [15] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||