Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (26): 4178-4184.doi: 10.3969/j.issn.2095-4344.2017.26.014
Previous Articles Next Articles
Lumbar interbody fusion with porous biphasic calcium phosphate enhanced by recombinant human bone morphogenetic protein-2/silk fibroin sustained-release microsphere in a sheep model
- 1People’s Hospital of Deyang City, Deyang 618000, Sichuan Province, China; 2the First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China
-
Received:2017-06-26Online:2017-09-18Published:2017-09-28 -
Contact:Chen Liang, Chief physician, Professor, Doctoral supervisor, the First Affiliated Hospital of Soochow University, Suzhou 215000, Jiangsu Province, China -
About author:Liu Hai-long, Master, Attending physician, People’s Hospital of Deyang City, Deyang 618000, Sichuan Province, China -
Supported by:the National Natural Science Foundation of China, No. 81071450, 81371930
CLC Number:
Cite this article
Liu Hai-long, Chen Liang, Gu Yong, Wang Chun-zeng, Liu Yue-hong.
share this article
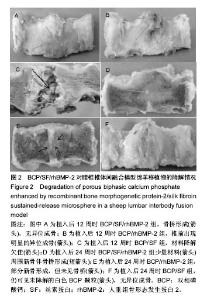
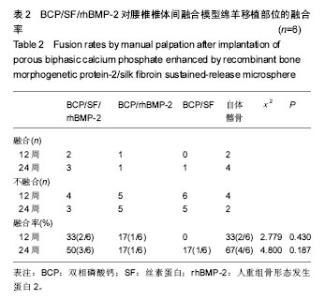
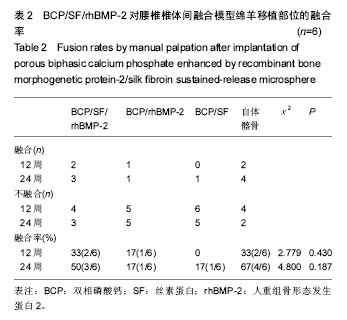
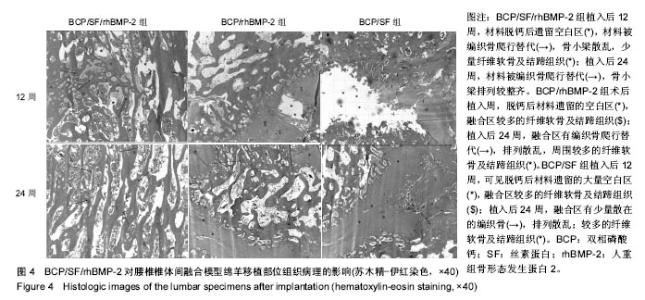
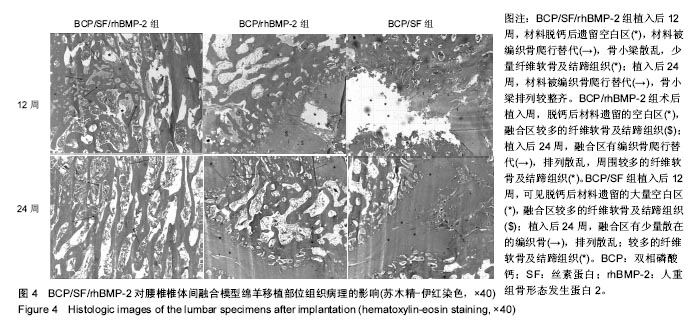
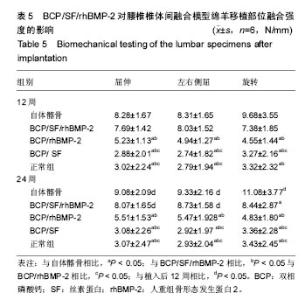
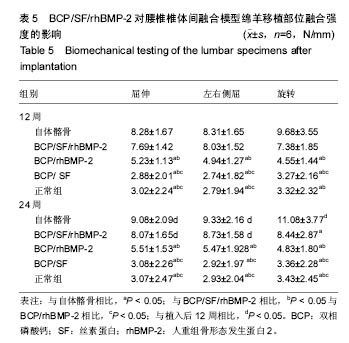
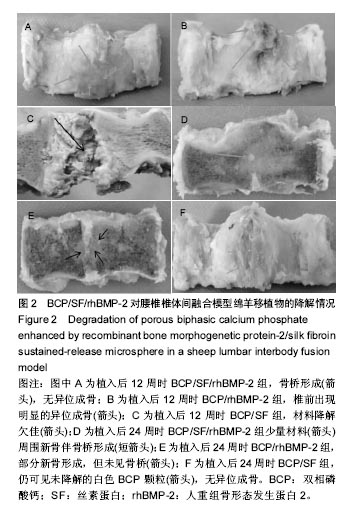
2.1 实验动物数量分析 各组动物均在术后2.0-3.0 h内清醒,并开始进食、饮水,4-6 h后逐渐站立行走,其中1只动物在植入后第5天出现纳食减退、精神萎靡,对症处理后症状无明显缓解,于植入后第7天死亡,考虑与术后肠麻痹有关,死亡动物随后给予补充。 2.2 大体情况 除死亡动物外各实验动物术后恢复顺利,未出现严重术后并发症。 2.3 材料整体情况 植入后12周:BCP/SF/rhBMP-2组材料降解良好,融合区已有较多的软骨样及骨样组织形成,部分标本椎体间已有骨性连接(图2A);BCP/rhBMP-2组材料降解良好,融合区有新生的骨样和纤维结缔组织,1例椎体前方有大块异位骨形成,但椎体间未形成连续的骨性连接(图2B);BCP/SF组材料降解明显欠佳,纤维结缔组织增生,椎体间无骨性连接(图2C)。 植入后24周:BCP/SF/rhBMP-2人工骨组可见材料降解良好,部分标本仅少量材料核残留,以材料核为中心,周围完整的骨组织已形成,椎体间骨性融合(图2D);BCP/rhBMP-2组可见材料降解良好,纤维结缔组织及骨样组织增生,椎体间有骨性结构形成,未连接(图2E);BCP/SF组仍可见白色材料残留,纤维结缔组织增生,椎体间未见骨性连接(图2F)。"
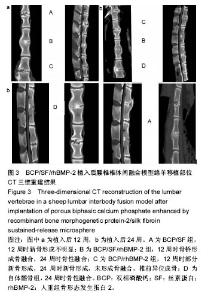
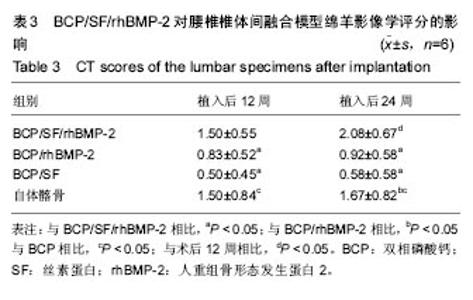
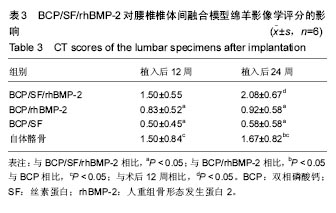
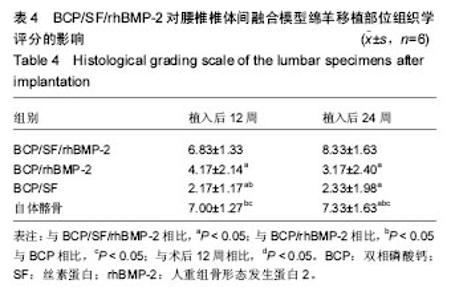
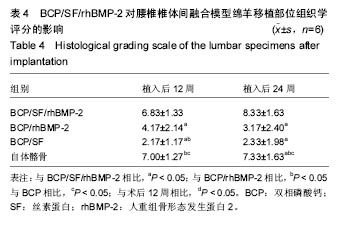
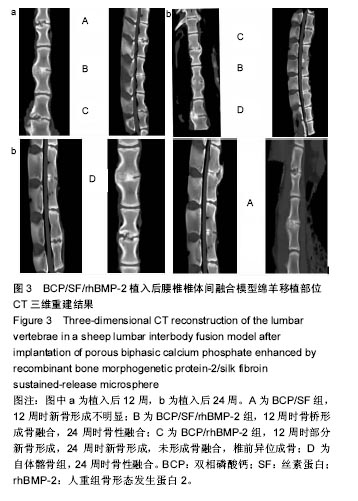
2.5 CT三维重建结果 植入后12周:2例BCP/SF/rhBMP-2人工骨组冠状面及矢状面重建均可见到,椎体间有骨性连接;BCP/rhBMP-2组冠状面及矢状面重建均可见到椎体间新生的骨性组织,1例形成骨性连接;BCP/SF组,冠状面及矢状面重建均未见到椎体间新生的骨性组织;2例自体髂骨组冠状面及矢状面重建均可见椎体间已有骨性连接(图3a)。 植入后24周:3例BCP/SF/rhBMP-2人工骨组冠状面及矢状面重建均可见到,椎体间有骨性连接;BCP/rhBMP-2组冠状面及矢状面重建均可见到椎体间有骨性结构,不连续,1例椎体前方有异位骨形成,1例形成骨性连接;BCP/SF组,冠状面及矢状面重建均可见到椎间少量新生骨组织,1例形成骨性连接;4例自体髂骨组冠状面及矢状面重建均可见椎间已有骨性连接(图3b)。植入后12和24周BCP/SF/ rhBMP-2及髂骨组的影像学评分优于其他2组;BCP/SF/ rhBMP-2植入后24周的影像学评分高于12周(表3)。"
| [1]Gruskay JA, Webb ML, Grauer JN. Methods of evaluating lumbar and cervical fusion. Spine J. 2014;14(3):531-539. [2]Malham GM, Parker RM, Ellis NJ, et al. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J Neurosurg Spine. 2014;21(6):851-860. [3]Liao JC, Chen WJ, Chen LH, et al. Surgical outcomes of degenerative spondylolisthesis with L5-S1 disc degeneration: comparison between lumbar floating fusion and lumbosacral fusion at a minimum 5-year follow-up. Spine (Phila Pa 1976). 2011;36(19):1600-1607. [4]Rezwan K, Chen QZ, Blaker JJ, et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18): 3413-3431. [5]Grauer JN, Beiner JM, Kwon B, et al. The evolution of allograft bone for spinal applications. Orthopedics. 2005; 28(6):573-577; quiz 578-579.[6]Majid K, Tseng MD, Baker KC, et al. Biomimetic calcium phosphate coatings as bone morphogenetic protein delivery systems in spinal fusion. Spine J. 2011;11(6):560-567. [7]Marie PJ, Debiais F, Haÿ E. Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling. Histol Histopathol. 2002;17(3):877-885. [8]Lad SP, Nathan JK, Boakye M. Trends in the use of bone morphogenetic protein as a substitute to autologous iliac crest bone grafting for spinal fusion procedures in the United States. Spine (Phila Pa 1976). 2011;36(4):E274-281.[9]Boerckel JD, Kolambkar YM, Dupont KM, et al. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32(22):5241-5251.[10]Autefage H, Briand-Mésange F, Cazalbou S, et al. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/beta-tricalcium phosphate porous ceramics. J Biomed Mater Res B Appl Biomater. 2009; 91(2): 706-715.[11]Chen L, Gu Y, Feng Y, et al. Bioactivity of porous biphasic calcium phosphate enhanced by recombinant human bone morphogenetic protein 2/silk fibroin microsphere. J Mater Sci Mater Med. 2014;25(7):1709-1719.[12]Gu Y, Chen L, Yang HL, et al. Evaluation of an injectable silk fibroin enhanced calcium phosphate cement loaded with human recombinant bone morphogenetic protein-2 in ovine lumbar interbody fusion. J Biomed Mater Res A. 2011;97(2): 177-185.[13]Murugan R, Ramakrishna S. Bioresorbable composite bone paste using polysaccharide based nano hydroxyapatite. Biomaterials. 2004;25(17):3829-3835.[14]Smucker JD, Bobst JA, Petersen EB, et al. B2A peptide on ceramic granules enhance posterolateral spinal fusion in rabbits compared with autograft. Spine (Phila Pa 1976). 2008;33(12):1324-1329. [15]Blattert TR, Delling G, Dalal PS, et al. Successful transpedicular lumbar interbody fusion by means of a composite of osteogenic protein-1 (rhBMP-7) and hydroxyapatite carrier: a comparison with autograft and hydroxyapatite in the sheep spine. Spine (Phila Pa 1976). 2002;27(23):2697-2705.[16]Lysack JT, Yen D, Dumas GA. In vitro flexibility of an experimental pedicle screw and plate instrumentation system on the porcine lumbar spine. Med Eng Phys. 2000;22(7): 461-468.[17]Abbah SA, Lam CX, Ramruttun AK, et al. Fusion performance of low-dose recombinant human bone morphogenetic protein 2 and bone marrow-derived multipotent stromal cells in biodegradable scaffolds: a comparative study in a large animal model of anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2011;36(21):1752-1759. [18]Smucker JD, Bobst JA, Petersen EB, et al. B2A peptide on ceramic granules enhance posterolateral spinal fusion in rabbits compared with autograft. Spine (Phila Pa 1976). 2008; 33(12):1324-1329. [19]Akita S, Fukui M, Nakagawa H, et al. Cranial bone defect healing is accelerated by mesenchymal stem cells induced by coadministration of bone morphogenetic protein-2 and basic fibroblast growth factor. Wound Repair Regen. 2004;12(2): 252-259.[20]Liu X, Ma PX. The nanofibrous architecture of poly(L-lactic acid)-based functional copolymers. Biomaterials. 2010; 31(2):259-269. [21]Lou T, Wang X, Song G. Fabrication of nano-fibrous poly(L-lactic acid) scaffold reinforced by surface modified chitosan micro-fiber. Int J Biol Macromol. 2013;61:353-358. [22]Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065-2073.[23]Liu X, Won Y, Ma PX. Porogen-induced surface modification of nano-fibrous poly(L-lactic acid) scaffolds for tissue engineering. Biomaterials. 2006;27(21):3980-3987. [24]Qiao C, Zhang K, Jin H, et al. Using poly(lactic-co-glycolic acid) microspheres to encapsulate plasmid of bone morphogenetic protein 2/polyethylenimine nanoparticles to promote bone formation in vitro and in vivo. Int J Nanomedicine. 2013;8:2985-2995. [25]Qiao C, Zhang K, Sun B, et al. Sustained release poly (lactic-co-glycolic acid) microspheres of bone morphogenetic protein 2 plasmid/calcium phosphate to promote in vitro bone formation and in vivo ectopic osteogenesis. Am J Transl Res. 2015;7(12):2561-2572. [26]Atluri K, Seabold D, Hong L, et al. Nanoplex-Mediated Codelivery of Fibroblast Growth Factor and Bone Morphogenetic Protein Genes Promotes Osteogenesis in Human Adipocyte-Derived Mesenchymal Stem Cells. Mol Pharm. 2015;12(8):3032-3042. [27]Jin H, Zhang K, Qiao C, et al. Efficiently engineered cell sheet using a complex of polyethylenimine-alginate nanocomposites plus bone morphogenetic protein 2 gene to promote new bone formation. Int J Nanomedicine. 2014;9: 2179-2190. [28]Xu X, Qiu S, Zhang Y, et al. PELA microspheres with encapsulated arginine-chitosan/pBMP-2 nanoparticles induce pBMP-2 controlled-release, transfected osteoblastic progenitor cells, and promoted osteogenic differentiation. Artif Cells Nanomed Biotechnol. 2017;45(2):330-339. [29]陈亮,顾勇,陈晓庆,等.丝素蛋白增强型磷酸钙复合rhBMP-2用于绵羊腰椎椎体间融合的实验研究[J].中华骨科杂志,2010, 30(7): 677-683.[30]Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med. 2008;2(2-3):81-96. |
| [1] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [2] | An Tiantian, Xu Yan, Chen Huiming, Zhang Xujing, Tan Hao. Preparation and performance of rifampicin-silk fibroin microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1515-1521. |
| [3] | Liu Xiaoyuan, Li Lei, Zhang Kai, Li Jun, Han Xiangzhen He Huiyu. Osteogenesis using bone marrow mesenchymal stem cell sheets combined with three-dimensional printed Cervus elaphus antler powder/silk fibroin/polyvinyl alcohol scaffold in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(34): 5420-5426. |
| [4] | Chen Zhenyu, Zhang Xiaoning, Luo Yuxin, Liang Jianwei, Yan Chi. Evaluation of silk fibroin/curcumin composite film for promoting wound healing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2554-2561. |
| [5] | Xu Changkui, Pu Xiaobing, Lu Yao, Chen Jiarong, Pan Lei. Safety and antibacterial properties of gentamicin-loaded silk fibroin in meniscus repair [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1545-1549. |
| [6] | Liu Xiaoyin, , Zhong Lin, Zheng Bo, Wei Pan, Dai Chen, Hu Liangcong, Wang Tiantian, Liang Xiaolong, Zhang Sai, Wang Xiaoli. Diffusion tensor imaging predicting locomotor function recovery with 3D printing scaffold after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4547-4554. |
| [7] | Zhang Xiaoyun, Chen Yueping, Song Shilei, Zhang Chi, Zhuo Yinghong, Yang Nan, Zhan Huasong, Xu Canhong. Good cell compatibility and permeability of silk fibroin/chitosan composite scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(16): 2544-2550. |
| [8] | She Rongfeng, Zhang Yi, Chen Long, Wang Yuanzheng, Zhang Bin, Huang Qixiang. Biocompatibility of tissue engineered cartilage constructed in vivo by silk fibroin-chitosan scaffold carrying bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(1): 27-32. |
| [9] | Wang Xuefeng, Shang Xifu . Curative effects of three filling materials in the treatment of osteoporotic thoracolumbar fractures [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(6): 863-869. |
| [10] | Liu Dan, Min Changqin, Lu Shuai, Chen Yue, Sun Yong. Osseointegration induced by beta-tricalcium phosphate loaded with advanced platelet-rich fibrin [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(6): 888-893. |
| [11] | Cheng Jian, Zhang Jun, Guan Jie, Zeng Junkai, Zhao Xin, Xie Youzhuan. Silver nanoparticle-doped tricalcium phosphate: in vitro and in vivo toxicity in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(6): 917-923. |
| [12] | Tang Xianneng, Chen Yueping, Zhang Xiaoyun. Clinical application of bone morphogenetic proteins in bone and cartilage tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(4): 591-596. |
| [13] | Liu Junyin, Feng Wei, Xie Yingchun, Li Yuwan, Zeng Jitao, Liu Ziming, Tu Xiaolin. Bone morphologic protein signaling pathway in bone regeneration and repair: accurate regulation and treatment targets [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(4): 606-612. |
| [14] | Chen Lei, Ge Weiming, Lü Linwei, Lei Ming, Teresa Zielińska, Xing Enhong. Mechanical properties of artificial cartilage scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(30): 4763-4768. |
| [15] | Sun Kai, Li Ruixin, Li Hao, Li Dong, Li Hui. Finite element analysis and demonstration of scaffold material stacking [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(30): 4787-4792. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||