Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (1): 27-32.doi: 10.3969/j.issn.2095-4344.1850
Previous Articles Next Articles
Biocompatibility of tissue engineered cartilage constructed in vivo by silk fibroin-chitosan scaffold carrying bone marrow mesenchymal stem cells
She Rongfeng, Zhang Yi, Chen Long, Wang Yuanzheng, Zhang Bin, Huang Qixiang
- Department of Orthopedics, Guizhou People’s Hospital, Guiyang 550002, Guizhou Province, China
-
Received:2019-06-24Revised:2019-07-02Accepted:2019-07-23Online:2020-01-08Published:2019-12-11 -
Contact:Zhang Yi, MD, Chief physician, Department of Orthopedics, Guizhou People’s Hospital, Guiyang 550002, Guizhou Province, China -
About author:She Rongfeng, Master, Attending physician, Department of Orthopedics, Guizhou People’s Hospital, Guiyang 550002, Guizhou Province, China -
Supported by:the Youth Fund of Guizhou Provincial People’s Hospital, No. GZSYQN[2015] 04; Guizhou Provincial Science and Technology Foundation Project, No. [2015]2096; Guizhou Science and Technology Plan Project, No. [2019]4446
CLC Number:
Cite this article
She Rongfeng, Zhang Yi, Chen Long, Wang Yuanzheng, Zhang Bin, Huang Qixiang. Biocompatibility of tissue engineered cartilage constructed in vivo by silk fibroin-chitosan scaffold carrying bone marrow mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(1): 27-32.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
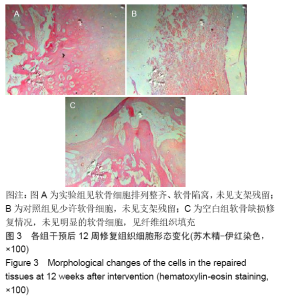
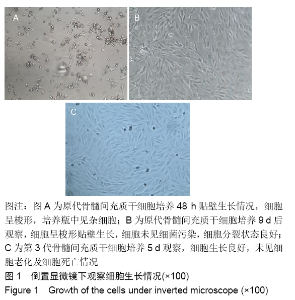
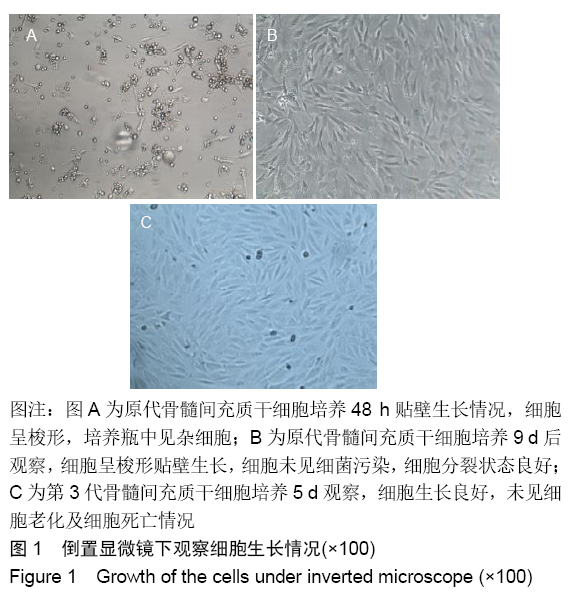
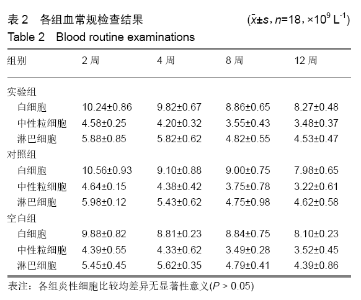
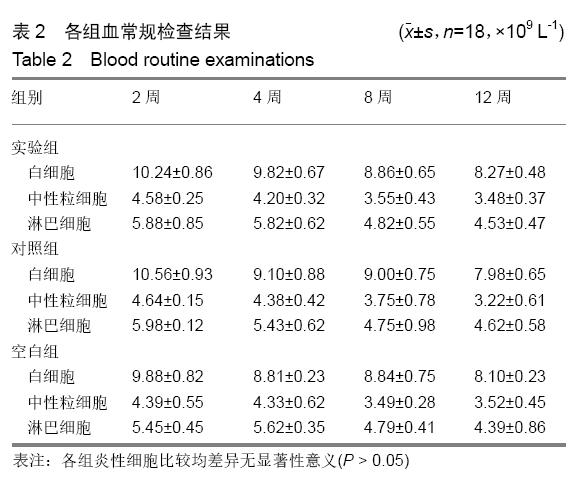
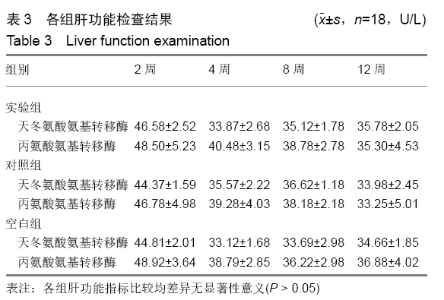
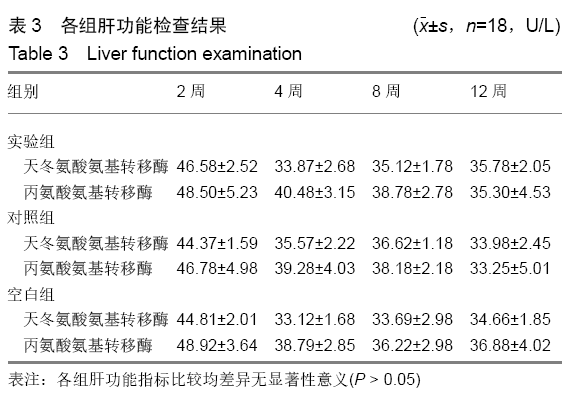
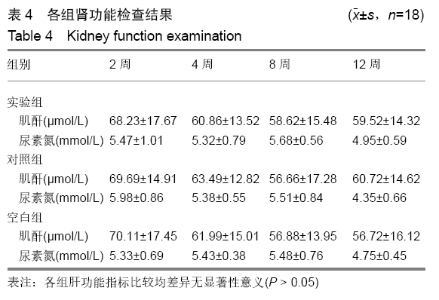
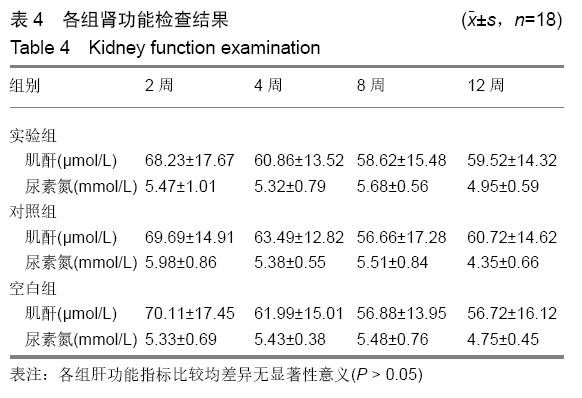
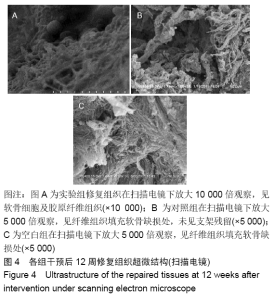
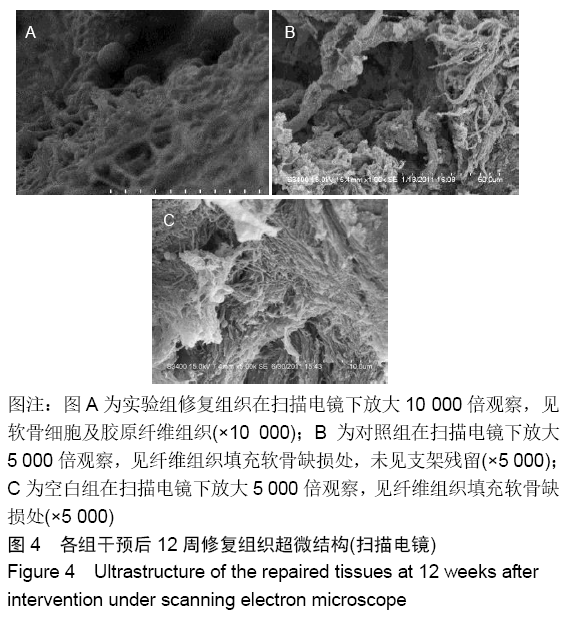
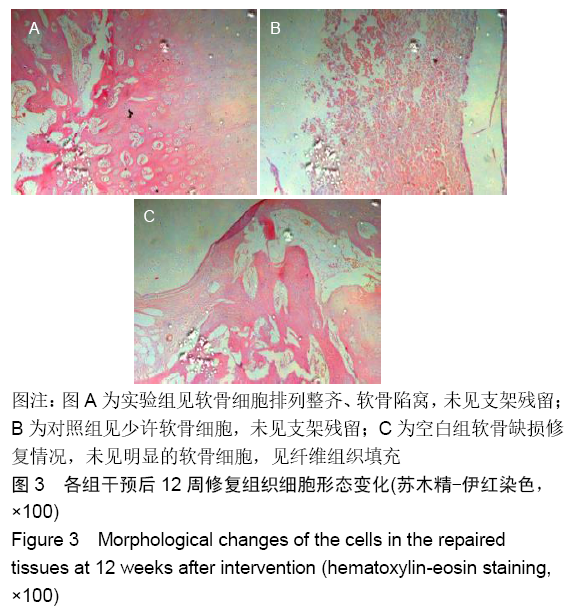
各组在干预后2,4,8,12周抽血查降钙素原结果均为阴性(< 0.05 μg/L),血沉和C-反应蛋白结果也均为阴性。各组进行修复膝关节腔抽取关节液白细胞、脓细胞进行检测,其结果均为阴性(0/HP)。 2.5 各组缺损部分组织学形态变化 干预后12周修复组织大体观:实验组软骨缺损处已修复,与周围正常软骨连接良好;对照组修复组织表面不规则,与周围正常软骨界限模糊;空白组软骨缺损处修复不良,缺损处仍有凹陷。 干预后12周修复组织苏木精-伊红染色:实验组缺损处由透明软骨组织修复,与周围正常组织连良好,见软骨陷窝,未见炎性细胞及支架材料,见图3A;对照组软骨缺损处见少许软骨组织修复,未见炎性细胞及支架材料,见图3B;空白组缺损处由纤维样组织填充,见图3C。 "
| [1] MARTÍN-HERNÁNDEZ C, FLORÍA-ARNAL LJ, GÓMEZ-BLASCO A, et al. Metaphyseal sleeves as the primary implant for the management of bone defects in total knee arthroplasty after post-traumatic knee arthritis. Knee. 2018;25(4):669-675. [2] ASHRAF S, KIM BJ, PARK S, et al. RHEB gene therapy maintains the chondrogenic characteristics and protects cartilage tissue from degenerative damage during experimental murine osteoarthritis. Osteoarthritis Cartilage. 2019 doi: 10.1016/j.joca.2019.05.024. [3] KHOSHBIN A, STAVRAKIS A, SHARMA A, et al. Patient-reported outcome measures of total knee arthroplasties for post-traumatic arthritis versus osteoarthritis: a short-term (5- to 10-year) retrospective matched cohort study. J Arthroplasty. 2019;34(5): 872-876. [4] ZIEGLER P, FRIEDERICHS J, HUNGERER S. Fusion of the subtalar joint for post-traumatic arthrosis: a study of functional outcomes and non-unions. Int Orthop. 2017;41(7):1387-1393. [5] DELIORMANLI AM, ATMACA H. Biological response of osteoblastic and chondrogenic cells to graphene-containing PCL/bioactive glass bilayered scaffolds for osteochondral tissue engineering applications. Appl Biochem Biotechnol. 2018;186(4):972-989. [6] RIBEIRO VP, DA SILVA MORAIS A, MAIA FR, et al. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018;72:167-181. [7] MIRAHMADI F, TAFAZZOLI-SHADPOUR M, SHOKRGOZAR MA, et al. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2013;33(8):4786-4794. [8] KIM CH, PARK SJ, YANG DH, et al. Chitosan for tissue engineering. Adv Exp Med Biol. 2018;1077:475-485. [9] VISHWANATH V, PRAMANIK K, BISWAS A. Optimization and evaluation of silk fibroin-chitosan freeze-dried porous scaffolds for cartilage tissue engineering application. J Biomater Sci Polym Ed. 2016;27(7):657-674. [10] DENG J, SHE R, HUANG W, et al. A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. J Mater Sci Mater Med. 2013;24(8):2037-2046. [11] ZENG S, LIU L, SHI Y, et al. Characterization of silk fibroin/chitosan 3D porous scaffold and in vitro cytology. PLoS One. 2015;10(6): e0128658. [12] KOPESKY PW, BYUN S, VANDERPLOEG EJ, et al. Sustained delivery of bioactive TGF-β1 from self-assembling peptide hydrogels induces chondrogenesis of encapsulated bone marrow stromal cells. J Biomed Mater Res A. 2014;102(5):1275-1285. [13] DUCRET M, FARGES JC, PASDELOUP M, et al. Phenotypic identification of dental pulp mesenchymal stem/stromal cells subpopulations with multiparametric flow cytometry. Methods Mol Biol. 2019;1922:77-90. [14] GOMATHYSANKAR S, HALIM AS, YAACOB NS, et al. Compatibility of porous chitosan scaffold with the attachment and proliferation of human adipose-derived stem cells in vitro. J Stem Cells Regen Med. 2016;12(2):79-86. [15] BRENNER JM, VENTURA NM, TSE MY, et al. Implantation of scaffold-free engineered cartilage constructs in a rabbit model for chondral resurfacing. Artif Organs. 2014;38(2):E21-E32. [16] AFEWERKI S, SHEIKHI A, KANNAN S, et al. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: towards natural therapeutics. Bioeng Transl Med. 2018;4(1): 96-115. [17] DIVAKAR P, MOODIE KL, DEMIDENKO E, et al. Quantitative evaluation of the in vivo biocompatibility and performance of freeze-cast tissue scaffolds. Biomed Mater. 2019 doi:10.1088/1748-605X/ab316a. [18] AGRAWAL P, PRAMANIK K, VISHWANATH V, et al. Enhanced chondrogenesis of mesenchymal stem cells over silk fibroin/ chitosan-chondroitin sulfate three dimensional scaffold in dynamic culture condition. J Biomed Mater Res B Appl Biomater. 2018; 106(7):2576-2587. [19] DEGERATU CN, MABILLEAU G, AGUADO E, et al. Polyhydroxyalkanoate (PHBV) fibers obtained by a wet spinning method: good in vitro cytocompatibility but absence of in vivo biocompatibility when used as a bone graft. Morphologie. 2019; 103(341 Pt 2):94-102. [20] TITORENCU I, ALBU MG, NEMECZ M, et al. Natural polymer-cell bioconstructs for bone tissue engineering. Curr Stem Cell Res Ther. 2017;12(2):165-174. [21] XIANG P, WANG SS, HE M, et al. The in vitro and in vivo biocompatibility evaluation of electrospun recombinant spider silk protein/PCL/gelatin for small caliber vascular tissue engineering scaffolds. Colloids Surf B Biointerfaces. 2018;163:19-28. [22] ZHUANG Y, ZHANG Q, FENG J, et al. The effect of native silk fibroin powder on the physical properties and biocompatibility of biomedical polyurethane membrane. Proc Inst Mech Eng H. 2017:231(4):337-346. [23] CAO L, LU C, WANG Q, et al. Biocompatibility and fabrication of RGO/chitosan film for cartilage tissue recovery. Environ Toxicol Pharmacol. 2017;54:199-203. [24] PERRIER-GROULT E, PÉRÈS E, PASDELOUP M, et al. Evaluation of the biocompatibility and stability of allogeneic tissue-engineered cartilage in humanized mice. PLoS One. 2019;14(5):e0217183. [25] SINGH BN, PRAMANIK K. Fabrication and evaluation of non-mulberry silk fibroin fiber reinforced chitosan based porous composite scaffold for cartilage tissue engineering. Tissue Cell. 2018;55:83-90. [26] ERICKSON AE, SUN J, LAN LEVENGOOD SK, et al. Chitosan-based composite bilayer scaffold as an in vitro osteochondral defect regeneration model. Biomed Microdevices. 2019;21(2):34. [27] XIAO H, HUANG W, XIONG K, et al. Osteochondral repair using scaffolds with gradient pore sizes constructed with silk fibroin, chitosan, and nano-hydroxyapatite. Int J Nanomedicine. 2019;14: 2011-2027. [28] ZHAO YH, NIU CM, SHI JQ, et al.Novel conductive polypyrrole/ silk fibroin scaffold for neural tissue repair.Neural Regen Res. 2018;13(8): 1455-1464. [29] 佘荣峰,邓江,黄文良,等.丝素蛋白/壳聚糖三维支架材料的制备方法[J].中国组织工程研究与临床康复,2011,15(47):8821-8824. [30] DENG J, SHE RF, HUANG WL, et al. Fibroin protein/chitosan scaffolds and bone marrow mesenchymal stem cells culture in vitro. Genet Mol Res. 2014;13(3):5745-5753. [31] 张敏波,彭齐峰,马亚萍,等.3D打印微小颗粒骨/聚乳酸-羟基乙酸共聚物支架材料的物理性能及其生物相容性[J].中国组织工程研究,2019, 23(14):2215-2222. [32] JONES JR, TSIGKOU O, COATES EE, et al. Extracellular matrix formation and mineralization on a phosphate-free porous bioactive glass scaffold using primary human osteoblast (HOB) cells. Biomaterials. 2007;28(9):1653-1663. [33] SEYEDMAJIDI M, HAGHANIFAR S, HAJIAN-TILAKI K, et al. Histopathological, histomorphometrical, and radiological evaluations of hydroxyapatite/bioactive glass and fluorapatite/bioactive glass nanocomposite foams as cell scaffolds in rat tibia: an in vivo study. Biomed Mater. 2018;13(2):025015. [34] WESTHAUSER F, SENGER AS, REIBLE B, et al. In vivo models for the evaluation of the osteogenic potency of bone substitutes seeded with mesenchymal stem cells of human origin: a concise review. Tissue Eng Part C Methods. 2017;23(12):881-888. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [3] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [4] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [5] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [6] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [7] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [8] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [9] | Zou Gang, Xu Zhi, Liu Ziming, Li Yuwan, Yang Jibin, Jin Ying, Zhang Jun, Ge Zhen, Liu Yi. Human acellular amniotic membrane scaffold promotes ligament differentiation of human amniotic mesenchymal stem cells modified by Scleraxis in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1037-1044. |
| [10] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [11] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [12] | Wu Gang, Chen Jianwen, Wang Shilong, Duan Xiaoran, Liu Haijun, Dong Jianfeng. Simple HyProCure subtalar stabilization in treatment of adolescent flexible flatfoot combined with painful accessory navicular bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 901-905. |
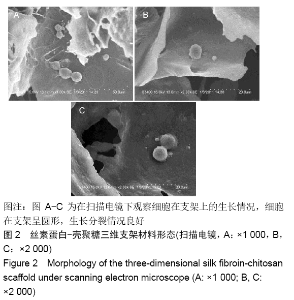
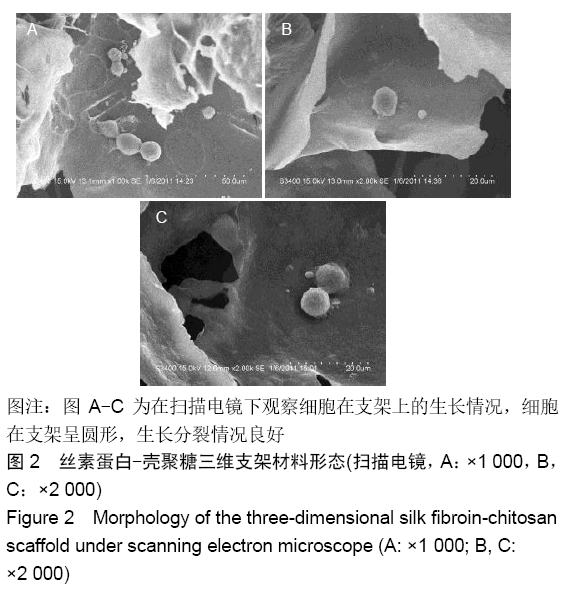
| [13] | Kong Lingbao, Lü Xin. Effect of implant selection and approach on support in the operation of posterolateral tibial plateau fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 942-947. |
| [14] | Liu Zhengpeng, Wang Yahui, Zhang Yilong, Ming Ying, Sun Zhijie, Sun He. Application of 3D printed interbody fusion cage for cervical spondylosis of spinal cord type: half-year follow-up of recovery of cervical curvature and intervertebral height [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 849-853. |
| [15] | Zhang Bin, Sun Lihua, Zhang Junhua, Liu Yusan, Cui Caiyun. A modified flap immediate implant is beneficial to soft tissue reconstruction in maxillary aesthetic area [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 707-712. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||