Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (20): 3234-3240.doi: 10.3969/j.issn.2095-4344.2017.20.019
Previous Articles Next Articles
Cell therapy is the most promising technique for degenerative intervertebral disc repair
Wang Yan-chao1, 2, Xi Zhi-peng2, 3, Xie Lin2, 3
- 1Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu Province, China; 2Nanjing Integrated Traditional Chinese and Western Medicine Hospital Affiliated to Nanjing University of Chinese Medicine, Nanjing 210028, Jiangsu Province, China; 3Jiangsu Traditional Chinese and Medicine Institute, Nanjing 210028, Jiangsu Province, China
-
Revised:2017-02-08Online:2017-07-18Published:2017-07-28 -
Contact:Xie Lin, Chief physician, Researcher, Nanjing Integrated Traditional Chinese and Western Medicine Hospital Affiliated to Nanjing University of Chinese Medicine, Nanjing 210028, Jiangsu Province, China; Jiangsu Traditional Chinese and Medicine Institute, Nanjing 210028, Jiangsu Province, China -
About author:Wang Yan-chao, Studying for master’s degree, Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu Province, China; Nanjing Integrated Traditional Chinese and Western Medicine Hospital Affiliated to Nanjing University of Chinese Medicine, Nanjing 210028, Jiangsu Province, China -
Supported by:the Science and Technology Project of Jiangsu Province, No. BK20151604
CLC Number:
Cite this article
Wang Yan-chao, Xi Zhi-peng, Xie Lin. Cell therapy is the most promising technique for degenerative intervertebral disc repair[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(20): 3234-3240.
share this article
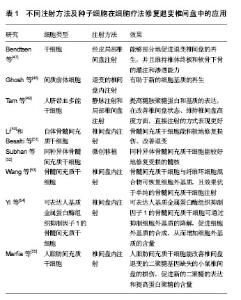
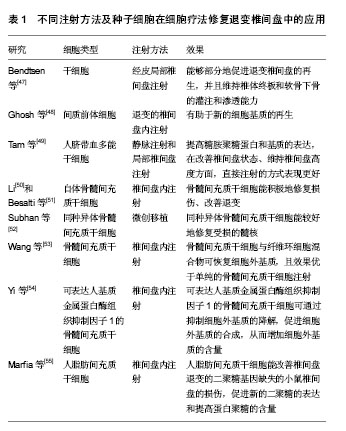
2.1 纳入文献基本情况 依据研究类型,将选取的文章分为实验室研究(46篇,占92%)和临床研究(4篇,占8%)。其中,临床研究数量较少,而实验室研究却占据了大多数。根据研究的方法不同,又将实验室研究细分为在体实验和离体实验。 2.2 体外实验 在实验室研究中,体外实验所占比重最大(36篇,占78%),涉及新方向、新理论及新机制等,像种子细胞的筛选、细胞共培养支架的发展、椎间盘微环境的探索、椎间盘器官培养、体外椎间盘退变环境的模拟等。 2.2.1 种子细胞 椎间盘退变的机制非常复杂,目前尚未完全明确,其中获得共识的观点是,髓核细胞的衰老和凋亡是椎间盘发生退变的重要原因[4]。而髓核中细胞含量少,髓核细胞的再生和增值能力有限,一旦发生退变,很难在自然条件下产生理想的修复效果和维持良好的功能[2],因此研究者试图找到一种合适的种子细胞来替代退变的髓核细胞从而延缓甚至逆转退变进程。 首先,研究者在探索中发现了新的细胞可以用来作为替代细胞。Yasen等[11]在研究中发现,兔椎间盘中存在祖细胞和细胞增殖现象,细胞增殖的数量和祖细胞标记物随年龄的增加而减少,因此,保持内源性祖细胞并刺激其增殖可以防止或抑制椎间盘的退变。Liu等[12]首次证明在人退变的软骨终板中存在间充质干细胞,该类细胞可诱导分化为成骨细胞、脂肪细胞、软骨细胞,在椎间盘修复方面存在潜力。Liu等[13]分离和描述了一个来源于兔纤维环组织的细胞亚群,发现这些细胞具有集落生成、自我更新和多向分化的能力。Cao等[14]研究发现,骨髓间充质干细胞可以通过上调转化生长因子β和下调核转录因子κB通路促进蛋白聚糖,Ⅱ型胶原蛋白和SOX-9基因的表达,进而延缓椎间盘的退变。Anderson等[15]将人脐血间充质干细胞注射进兔椎间盘中,发现该细胞具有良好的生存能力和软骨分化能力。 其次,新的替代细胞是否具有髓核细胞的特性,可以真正地替代髓核细胞的功能?研究者对其进行的不懈的努力。Liu等[16]的研究证实,在灭活的猪髓核基质的影响下,人诱导多能干细胞可向脊索细胞分化。Chen等[17]和Liu等[18]通过对诱导多能干细胞的体外培养与鉴定,发现诱导多能干细胞具有分化为髓核样细胞的潜能。Ni等[19]成功分离了胚胎来源的间充质干细胞,并发现其在缺氧条件下培养,类髓核细胞标记物的表达明显增加。Jin等[20]利用转化生长因子β对脂肪间充质干细胞进行诱导,发现椎间盘样细胞标志物的表达及细胞外基质明显增高。Sun等[21]通过机械压力实验发现,脂肪间充质干细胞可以阻止机械压力造成的髓核细胞的死亡和退化。Han等[22]研究发现,在酸性条件下,髓核间充质干细胞在修复退变的椎间盘方面,比脂肪间充质干细胞表现的更好。Shi等[23]首次证明了椎间盘区域潜在干细胞龛来源干细胞的存在,并发现该细胞属于间充质干细胞家族,拥有较骨髓间充质干细胞更好的成骨细胞和成软骨细胞的能力。目前,对骨髓间充质干细胞和脂肪间充质干细胞的研究较为成熟,同时诱导多能干细胞的研究队伍也在不断壮大,对椎间盘区域潜在干细胞的探索正在逐步兴起。 2.2.2 细胞培养支架 支架是细胞体外共培养体系中非常重要的组成部分。Bertolo等[24]比较了马胶原蛋白、猪胶原蛋白、凝胶和聚氨基葡萄糖的支架对骨髓间充质干细胞在体外髓核样分化的情况,发现其中胶原蛋白支架的效果更好。而Meitsch等[25]研究发现三维微球培养系统比海藻盐酸纳更有利于人骨髓间充质干细胞向软骨细胞分化。Potier等[26]在一个简化的体外三维培养体系中将骨髓间充质干细胞与髓核细胞进行共培养,发现2种细胞能够相互刺激。Pirvu等[27]将骨髓间充质干细胞注入聚三亚甲基碳酸酯中用于椎间盘的修复,发现该支架在恢复椎间盘高度和促进干细胞向椎间盘细胞分化方面表现良好。Naqvi和Buckley[28]则发现相比于壳聚糖水凝胶,海藻酸钠能更好地支持髓核细胞和骨髓干细胞中硫酸化糖胺聚糖的积累和Ⅱ型胶原蛋白的沉积。Bian等[29]构建了一种包含KLD-12多肽/转化生长因子β1的纳米纤维凝胶的组织工程支架,并发现其可诱导间充质干细胞分化为髓核样细胞。Gupta等[30]将光交联羟甲基纤维素水凝胶用于体外细胞培养,发现它能够为人间充质干细胞产生髓核样细胞外基质提供支持。Nair等[31]研制了一种由壳聚糖聚乙烯醇与硫酸软骨素纳米粒子构成的复合水凝胶,其有利于保持大鼠脂肪间充质干细胞的活力和黏附能力,并能增强间充质干细胞的存活率和成软骨分化能力。伴随着新材料的出现,支架的发展非常迅速,从传统的海藻盐溶液、水凝胶,到胶原蛋白、三维纳米材料,相信未来会出现更多更好的支架为细胞的培养和环境提供支持。 2.2.3 椎间盘微环境 椎间盘内处于低氧、低营养、高渗透压、高机械强度的环境中,研究者们渴望通过模拟椎间盘的微环境,并观察环境改变对细胞代谢的影响,从而揭示退变的机制。Salvatierra等[32]探讨了纤维环细胞和髓核细胞在动态压缩下的能量代谢,发现压缩能增加2种细胞中三磷酸腺苷的释放,从而影响椎间盘细胞的能量代谢,其中对纤维环细胞的作用更大。Dai等[33]在研究中发现,动态压缩可以促进脂肪间充质干细胞的增殖,并能诱导其分化成为髓核样细胞。Hu等[34]评估了月经血干细胞分别在正常和低氧条件下向髓核样细胞分化的能力,发现低氧能增强髓核特征标记物基因的表达。Ni等[19]研究了缺氧对人胎盘间充质干细胞向髓核样细胞分化的影响,证明缺氧条件可以促进其向髓核样细胞的分化和增殖。Arkesteijn等[35]将犬脊索细胞、髓核细胞与间充质干细胞置于缺氧和高渗透压条件下共同培养,并没有观察到脊索细胞在该条件下的再生潜能。Han等[22]在体外酸性条件下对髓核间充质干细胞和脂肪间充质干细胞在增殖和基质代谢方面进行了研究,发现酸性环境是椎间盘细胞再生的主要障碍,髓核间充质干细胞对酸性抑制作用的敏感度更低。Liang等[36]分别调查了在正常和退变椎间盘化学微环境下,人脂肪间充质干细胞在椎间盘再生方面的可行性、细胞增殖及主要基质蛋白的表达情况,发现低糖是一个积极的因素,但高渗透压和低pH值是影响其生存和生物学行为的有害因素。Naqvi等[37]比较了髓核细胞和骨髓干细胞在氧气和葡萄糖浓度改变的典型椎间盘微环境里关键基质蛋白的产量及活力,结果表明,低糖和低氧可能是影响干细胞生存和生物学行为的关键。 2.2.4 器官培养和相关因子 研究者希望通过建立离体椎间盘器官培养体系来模拟椎间盘退变的环境变化及相关疗法的筛选,从而提高实验效率、降低动物实验的数量。Pirvu等[27]将牛尾椎间盘分离至体外,利用动态压力制成纤维环破裂模型。Teixeira等[38]在体外利用针刺和白细胞介素1β建立起促炎/退行性椎间盘器官培养模型,用于抗炎药物和疗法的筛选。Furtwängler等[39]通过向牛尾椎间盘中注射蛋白水解的基质金属蛋白酶3、聚蛋白多糖酶(ADAMTS-4)及人高温丝氨酸蛋白酶A1,发展了一个椎间盘退变器官培养模型。Pattappa等[40]通过体外诱导牛尾椎间盘退变,发现趋化因子配体5可能是一种由椎间盘细胞产生并释放的关键性趋化因子。 其次,关于相关因子的研究数量相当庞大,大量的研究者希望从中找到导致椎间盘退变的分子机制。Tiaden等[41]研究证实,HTRA1可通过水解纤维连接蛋白,继发地活化椎间盘细胞从而促进椎间盘退变。Jin等[20]的研究发现,转化生长因子β3可促进脂肪间充质干细胞向椎间盘样细胞分化及相关标志物,如蛋白聚糖、Ⅱ型胶原蛋白和SOX-9的表达。Hu等[42]在研究椎间盘退变的实验中发现,白细胞介素1β能刺激髓核细胞,使其能够吸引和介导骨髓间充质干细胞的迁移。Luo等[43]成功地将腺病毒介导的生长分化因子5转染了人退变髓核细胞,发现其能有效地促进细胞外基质中蛋白聚糖和Ⅱ型胶原蛋白的分泌,在一定程度上促进退变髓核细胞的生长。Clarke等[44]研究的结果表明,白生长分化因子6能刺激脂肪间充质干细胞分化为髓核样细胞表型,从而导致一个富含蛋白多糖的基质。De Vries等[45]在研究细胞外基质时发现,来源于富含脊索细胞的髓核组织的条件培养基能够刺激髓核细胞和骨髓间充质干细胞基质的生产,并能直接促进髓核细胞向更健康的细胞表型转变。Gupta等[30]在新型支架的研究中发现,短期的转化生长因子β3干预可促进组织的成熟。Suzuki等[46]发现氧化应激可导致椎间盘的退变。 2.3 体内实验 研究者们依据基础实验的丰硕成果,将细胞注射疗法应用于动物实验,获得了许多令人满意的成果,为临床实验打下了坚实的基础。 2.3.1 注射方法及种子细胞 Bendtsen等[47]经皮局部椎间盘注射干细胞和水凝胶,发现该疗法能够部分地促进退变椎间盘的再生,并且维持椎体终板和软骨下骨的灌注和渗透能力。Ghosh等[48]的研究证实,在退变的椎间盘内注射间质前体细胞有助于新的细胞基质的再生。Tam等[49]将人脐血多能干细胞分别以静脉注射和局部椎间盘注射的方法植入大鼠椎间盘退变模型中,2种方法都能提高糖胺聚糖蛋白和基质的表达,在改善椎间盘状态、维持椎间盘高度方面,直接注射的方式表现更好。Li[50]和Besalti等[51]通过注射自体骨髓间充质干细胞,来探索其在治疗椎间盘退变及相关疾病中的意义,发现骨髓间充质干细胞能积极地修复损伤、改善退变。Subhan等[52]在开发干细胞移植微创技术时,利用的是同种异体骨髓间充质干细胞,能较好地修复受损的髓核。Wang等[53]将骨髓间充质干细胞与纤维环细胞混合物注入椎间盘中,结果发现混合物可恢复细胞外基质,且效果优于单纯的骨髓间充质干细胞注射。 Yi等[54]利用病毒转染技术创造出可表达人基质金属蛋白酶组织抑制因子1的骨髓间充质干细胞,将其移植入退变的椎间盘后发现,该细胞可通过抑制细胞外基质的降解,促进细胞外基质的合成,从而增加细胞外基质的含量。Marfia等[55]将人脂肪间充质干细胞植入椎间盘退变的二聚糖基因缺失的小鼠中,结果发现其能改善椎间盘的损伤,促进新的二聚糖的表达和提高蛋白聚糖的含量(表1)。"
| [1] Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859): 2163-2196. [2] Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057-1070. [3] Chan SC, Walser J, Käppeli P, et al. Region specific response of intervertebral disc cells to complex dynamic loading: an organ culture study using a dynamic torsion-compression bioreactor. PLoS One. 2013;8(8):e72489. [4] Anderson DG, Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5(6 Suppl): 260S-266S.[5] Mochida J, Nishimura K, Nomura T, et al. The importance of preserving disc structure in surgical approaches to lumbar disc herniation. Spine (Phila Pa 1976). 1996;21(13):1556-1563; discussion 1563-1564.[6] Mochida J, Toh E, Nomura T, et al. The risks and benefits of percutaneous nucleotomy for lumbar disc herniation. A 10-year longitudinal study. J Bone Joint Surg Br. 2001;83(4): 501-505.[7] Alini M, Roughley PJ, Antoniou J, et al. A biological approach to treating disc degeneration: not for today, but maybe for tomorrow. Eur Spine J. 2002;11 Suppl 2:S215-220.[8] An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine (Phila Pa 1976). 2003;28(15 Suppl): S86-92.[9] Hohaus C, Ganey TM, Minkus Y, et al. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008; 17 Suppl 4:492-503. [10] Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24(1):5-21. [11] Yasen M, Fei Q, Hutton WC, et al. Changes of number of cells expressing proliferation and progenitor cell markers with age in rabbit intervertebral discs. Acta Biochim Biophys Sin (Shanghai). 2013;45(5):368-376. [12] Liu LT, Huang B, Li CQ, et al. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS One. 2011;6(10):e26285. [13] Liu C, Guo Q, Li J, et al. Identification of rabbit annulus fibrosus-derived stem cells. PLoS One. 2014;9(9):e108239. [14] Cao C, Zou J, Liu X, et al. Bone marrow mesenchymal stem cells slow intervertebral disc degeneration through the NF-κB pathway. Spine J. 2015;15(3):530-538. [15] Anderson DG, Markova D, An HS, et al. Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair. Am J Phys Med Rehabil. 2013;92(5):420-429. [16] Liu Y, Fu S, Rahaman MN, et al. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res A. 2015; 103(3):1053-1059. [17] Chen J, Lee EJ, Jing L, et al. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS One. 2013;8(9):e75548. [18] Liu K, Chen Z, Luo XW, et al. Determination of the potential of induced pluripotent stem cells to differentiate into mouse nucleus pulposus cells in vitro. Genet Mol Res. 2015;14(4):12394-12405. [19] Ni L, Liu X, Sochacki KR, et al. Effects of hypoxia on differentiation from human placenta-derived mesenchymal stem cells to nucleus pulposus-like cells. Spine J. 2014; 14(10):2451-2458. [20] Jin ES, Min J, Jeon SR, et al. Analysis of molecular expression in adipose tissue-derived mesenchymal stem cells : prospects for use in the treatment of intervertebral disc degeneration. J Korean Neurosurg Soc. 2013;53(4):207-212. [21] Sun Z, Luo B, Liu ZH, et al. Adipose-derived stromal cells protect intervertebral disc cells in compression: implications for stem cell regenerative disc therapy. Int J Biol Sci. 2015; 11(2):133-143. [22] Han B, Wang HC, Li H, et al. Nucleus pulposus mesenchymal stem cells in acidic conditions mimicking degenerative intervertebral discs give better performance than adipose tissue-derived mesenchymal stem cells. Cells Tissues Organs. 2014;199(5-6):342-352. [23] Shi R, Wang F, Hong X, et al. The presence of stem cells in potential stem cell niches of the intervertebral disc region: an in vitro study on rats. Eur Spine J. 2015;24(11):2411-2424. [24] Bertolo A, Mehr M, Aebli N, et al. Influence of different commercial scaffolds on the in vitro differentiation of human mesenchymal stem cells to nucleus pulposus-like cells. Eur Spine J. 2012;21 Suppl 6:S826-838. [25] Mietsch A, Neidlinger-Wilke C, Schrezenmeier H, et al. Evaluation of platelet-rich plasma and hydrostatic pressure regarding cell differentiation in nucleus pulposus tissue engineering. J Tissue Eng Regen Med. 2013;7(3):244-252. [26] Potier E, Ito K. Can notochordal cells promote bone marrow stromal cell potential for nucleus pulposus enrichment? A simplified in vitro system. Tissue Eng Part A. 2014;20(23-24): 3241-3251.[27] Pirvu T, Blanquer SB, Benneker LM, et al. A combined biomaterial and cellular approach for annulus fibrosus rupture repair. Biomaterials. 2015;42:11-19. [28] Naqvi SM, Buckley CT. Differential response of encapsulated nucleus pulposus and bone marrow stem cells in isolation and coculture in alginate and chitosan hydrogels. Tissue Eng Part A. 2015;21(1-2):288-299. [29] Bian Z, Sun J. Development of a KLD-12 polypeptide/ TGF-β1-tissue scaffold promoting the differentiation of mesenchymal stem cell into nucleus pulposus-like cells for treatment of intervertebral disc degeneration. Int J Clin Exp Pathol. 2015;8(2):1093-1103. [30] Gupta MS, Nicoll SB. Duration of TGF-β3 Exposure Impacts the Chondrogenic Maturation of Human MSCs in Photocrosslinked Carboxymethylcellulose Hydrogels. Ann Biomed Eng. 2015;43(5):1145-1157. [31] Nair MB, Baranwal G, Vijayan P, et al. Composite hydrogel of chitosan-poly(hydroxybutyrate-co-valerate) with chondroitin sulfate nanoparticles for nucleus pulposus tissue engineering. Colloids Surf B Biointerfaces. 2015;136:84-92. [32] Salvatierra JC, Yuan TY, Fernando H, et al. Difference in Energy Metabolism of Annulus Fibrosus and Nucleus Pulposus Cells of the Intervertebral Disc. Cell Mol Bioeng. 2011;4(2):302-310.[33] Dai J, Wang H, Liu G, et al. Dynamic compression and co-culture with nucleus pulposus cells promotes proliferation and differentiation of adipose-derived mesenchymal stem cells. J Biomech. 2014;47(5):966-972. [34] Hu X, Zhou Y, Zheng X, et al. Differentiation of menstrual blood-derived stem cells toward nucleus pulposus-like cells in a coculture system with nucleus pulposus cells. Spine (Phila Pa 1976). 2014;39(9):754-760. [35] Arkesteijn IT, Smolders LA, Spillekom S, et al. Effect of coculturing canine notochordal, nucleus pulposus and mesenchymal stromal cells for intervertebral disc regeneration. Arthritis Res Ther. 2015;17:60. [36] Liang C, Li H, Tao Y, et al. Responses of human adipose- derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med. 2012;10:49. [37] Naqvi SM, Buckley CT. Extracellular matrix production by nucleus pulposus and bone marrow stem cells in response to altered oxygen and glucose microenvironments. J Anat. 2015;227(6):757-766.[38] Teixeira GQ, Boldt A, Nagl I, et al. A Degenerative/ Proinflammatory Intervertebral Disc Organ Culture: An Ex Vivo Model for Anti-inflammatory Drug and Cell Therapy. Tissue Eng Part C Methods. 2016;22(1):8-19. [39] Furtwängler T, Chan SC, Bahrenberg G, et al. Assessment of the matrix degenerative effects of MMP-3, ADAMTS-4, and HTRA1, injected into a bovine intervertebral disc organ culture model. Spine (Phila Pa 1976). 2013;38(22): E1377-1387. [40] Pattappa G, Peroglio M, Sakai D, et al. CCL5/RANTES is a key chemoattractant released by degenerative intervertebral discs in organ culture. Eur Cell Mater. 2014;27:124-136; discussion 136.[41] Tiaden AN, Klawitter M, Lux V, et al. Detrimental role for human high temperature requirement serine protease A1 (HTRA1) in the pathogenesis of intervertebral disc (IVD) degeneration. J Biol Chem. 2012;287(25):21335-21345. [42] Hu J, Deng G, Tian Y, et al. An in vitro investigation into the role of bone marrow?derived mesenchymal stem cells in the control of disc degeneration. Mol Med Rep. 2015;12(4): 5701-5708.[43] Luo XW, Liu K, Chen Z, et al. Adenovirus-mediated GDF-5 promotes the extracellular matrix expression in degenerative nucleus pulposus cells. J Zhejiang Univ Sci B. 2016;17(1): 30-42.[44] Clarke LE, McConnell JC, Sherratt MJ, et al. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16(2):R67. [45] de Vries SA, Potier E, van Doeselaar M, et al. Conditioned medium derived from notochordal cell-rich nucleus pulposus tissue stimulates matrix production by canine nucleus pulposus cells and bone marrow-derived stromal cells. Tissue Eng Part A. 2015;21(5-6):1077-1084. [46] Suzuki S, Fujita N, Hosogane N, et al. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther. 2015;17:316.[47] Bendtsen M, Bünger CE, Zou X, et al. Autologous stem cell therapy maintains vertebral blood flow and contrast diffusion through the endplate in experimental intervertebral disc degeneration. Spine (Phila Pa 1976). 2011;36(6):E373-379. [48] Ghosh P, Moore R, Vernon-Roberts B, et al. Immunoselected STRO-3+ mesenchymal precursor cells and restoration of the extracellular matrix of degenerate intervertebral discs. J Neurosurg Spine. 2012;16(5):479-488. [49] Tam V, Rogers I, Chan D, et al. A comparison of intravenous and intradiscal delivery of multipotential stem cells on the healing of injured intervertebral disk. J Orthop Res. 2014; 32(6):819-825. [50] Li YY, Diao HJ, Chik TK, et al. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation. Tissue Eng Part A. 2014;20(9-10):1379-1391. [51] Besalti O, Can P, Akpinar E, et al. Intraspinal Transplantation of Autologous Neurogenically-Induced Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Paraplegic Dogs without Deep Pain Perception Secondary to Intervertebral Disk Disease. Turk Neurosurg. 2015;25(4): 625-632.[52] Subhan RA, Puvanan K, Murali MR, et al. Fluoroscopy assisted minimally invasive transplantation of allogenic mesenchymal stromal cells embedded in HyStem reduces the progression of nucleus pulposus degeneration in the damaged ntervertebral [corrected] disc: a preliminary study in rabbits. ScientificWorldJournal. 2014;2014:818502. [53] Wang YH, Yang B, Li WL, et al. Effect of the mixture of bone marrow mesenchymal stromal cells and annulus fibrosus cells in repairing the degenerative discs of rabbits. Genet Mol Res. 2015;14(1):2365-2373. [54] Yi Z, Guanjun T, Lin C, et al. Effects of Transplantation of hTIMP1-Expressing Bone Marrow Mesenchymal Stem Cells on the Extracellular Matrix of Degenerative Intervertebral Discs in an in vivo Rabbit Model. Spine (Phila Pa 1976). 2014. in press.[55] Marfia G, Campanella R, Navone SE, et al. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration. Arthritis Res Ther. 2014;16(5):457. [56] Vadalà G, Sowa G, Hubert M, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012; 6(5):348-355. [57] Orozco L, Soler R, Morera C, et al. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92(7):822-828.[58] Pettine KA, Murphy MB, Suzuki RK, et al. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015;33(1):146-156.[59] Pang X, Yang H, Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician. 2014; 17(4):E525-530.[60] Mochida J, Sakai D, Nakamura Y, et al. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cell Mater. 2015;29:202-212; discussion 212. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1530-1536. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||