Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (2): 296-301.doi: 10.3969/j.issn.2095-4344.2017.02.024
Previous Articles Next Articles
Application of silicon in biomaterials: an increase in bioactivity but with no change in mechanical performance
- Department of Orthopaedics, Affiliated Putuo Hospital of Shanghai University of Traditional Chinese Medicine, Shanghai 200062, China
-
Received:2016-10-19Online:2017-01-18Published:2017-02-27 -
Contact:Ji Bin, Chief physician, Master’s supervisor, Department of Orthopaedics, Affiliated Putuo Hospital of Shanghai University of Traditional Chinese Medicine, Shanghai 200062, China -
About author:Zhang Shai-lin, M.D., Attending physician, Department of Orthopaedics, Affiliated Putuo Hospital of Shanghai University of Traditional Chinese Medicine, Shanghai 200062, China -
Supported by:the Self-Innovation Project of Health System in Shanghai Putuo District, No. 2013PTKW015
CLC Number:
Cite this article
Zhang Shai-lin, Cheng Xiang-yu, Ji Bin.
share this article
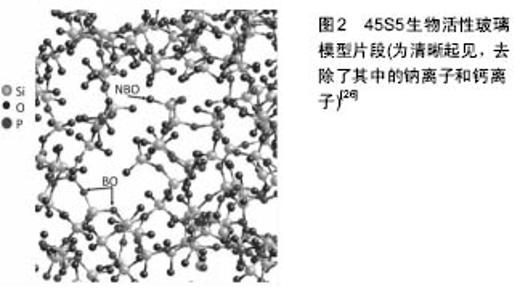
2.1 硅在骨骼生物学中的作用 Carlisle[1-2]和Schwarz[3]发现,硅是正常生长发育的必须元素,饮食中缺少硅可导致异常的骨和软骨形成。Carlisle证明了用添加硅的食物(含有28 mg/L硅的硅酸钠)喂养小鸡可促进骨生长、增加关节软骨的量、增加骨质含水量、骨盐生化改变、氨基己糖和胶原含量[4-5]。电子探针分析表明,硅主要集中于发育中骨的生长部位,如骨骺生长板[6-7],此处是成骨活性最高的部位。 成骨细胞中硅与镁和磷的数量相近[5],分布于线粒体和其他细胞内的细胞器[6],通过对腹腔内注射硅胶引起肾病的大鼠线粒体进行研究进一步证实了这一点。硅位于线粒体内,形成密集的直径4.0-5.0 nm大小的球体,并进一步形成直径15-120 nm的聚集体[8]。 体外研究表明,添加浓度为5-50 μmol/L原硅酸的人成骨细胞Ⅰ型胶原蛋白表达显著增加[9],原硅酸浓度为10-20 μmol/L时,Ⅰ型胶原蛋白合成增加[10]。与缺少硅的小鸡相比,食物中添加硅可使发育中的小鸡喂养 12 d后骨胶原蛋白含量增加100%[4]。 研究表明,细胞内信号转导、基因表达和蛋白质合成的改变,至少可以部分由离子取代辅因子解释。例如,硅铝复合物阻止铝离子与铁离子竞争涉及胶原蛋白合成和功能的分子结合位点,如脯氨酰羟化酶[11-12]。Carlisle和Suchil[13]的研究表明,添加硅酸盐可使氨基己糖、脯氨酸和总蛋白含量增加,特别是有抗坏血酸存在时。 Lia和Changa[14]发现,硅酸钙释放硅离子可上调成纤维细胞和内皮细胞的血管内皮生长因子的表达,血管内皮生长因子可上调骨形态发生蛋白的表达[15],因此,以往的研究某种程度上阐明了硅在骨骼和血管形成中的作用。硅还能抑制巨噬细胞和破骨细胞活性[16],也可能是通过与抗坏血酸相互作用[17]。 2.2 羟基磷灰石中的硅取代 骨盐一般被称为羟基磷灰石,骨中的钙/磷摩尔比与纯羟基磷灰石略有不同[18]。这是由于有许多其他原子和小分子取代基质中钙、磷离子所致,如镁和锶取代钙[19],健康人骨中发现的取代分子大多数为碳酸盐、钠和镁[20]。除了碳酸盐,羟基残基可以被氯化物和氟化物取代[20]。各种不同的离子取代是由于羟基磷灰石作为一种矿物质的相对不稳定以及其与细胞外液接触的晶体表面积较大所致[20]。少量硅和其他元素的离子取代已被证明对热稳定性、溶解度、破骨细胞及成骨细胞的体外和体内反应具有显著影响[21]。 对患者饮食进行的研究表明,增加人体食物中硅含量,除了绝经后妇女,所有受试者髋部皮质骨的骨密度都增加了[22]。最高摄入量组(Si > 40 mg/d)和最低摄入量组(Si < 14 mg /d)髋部骨密度差异较大(达10%),这为明确可生理性刺激骨生长的生物可利用硅的浓度提供了有用的数据。有趣的是,虽然脊椎松质骨通常被认为代谢上更敏感,研究者发现皮质骨受硅的影响很大,脊椎松质骨所受的影响却较小。研究者推断这可能是因为硅的作用是促进骨的合成代谢,而不是抑制破骨细胞的骨吸收作用[22]。 2.3 细胞外基质中的硅 Schwarz[2]的研究表明,硅对细胞外基质中的糖胺聚糖的正常形成至关重要,有利于稳定复合多糖结构,通过硅醇键形成交联,从而调节这些分子的结构和功能。同时,Carlisle的研究表明,孵育12 d后,和缺少硅的鸡胚相比,添加了硅的鸡胚骨骼胶原含量增加了100%[4]。 有研究者利用硅缺乏的症状作为判断需要硅的生物学过程的一种方法。在这些研究中,骨骼发育异常成为最主要的症状[23]。对缺乏硅的鸡骨骼发育进行的研究表明可导致腿骨周径减小,骨皮质变薄和弹性降低[24]。分别采用硅充足和硅缺乏食物喂养小鸡4周,可发现硅缺乏对发育显著的生理影响。硅缺乏的鸡头颅形状异常[2],看上去比正常颅骨平坦,缺少典型的条纹状骨小梁基质,代之的是紊乱的、结节状的不成熟骨的生长特性。 Carlisle[4]发现,硅位于年轻小鼠和大鼠的骨生长活跃区,在骨样组织中随着钙离子浓度增加同时增加。当骨骼成熟后,钙水平接些完全钙化骨,硅的水平减少。这表明,硅可能在有机基质发展和钙化起始中发挥作用。硅缺乏的动物中糖胺聚糖和胶原蛋白的浓度降低,而骨矿物成分基本未受影响,进一步证明了硅的这一作用。研究发现这些多糖的提取物在有些组织中与硅化学上相关[2],表明细胞外基质中羟基磷灰石晶体形成前,硅可能发挥协调糖蛋白与胶原磷酸化反应的作用。 2.4 生物活性玻璃和成骨细胞对硅酸盐生物材料的反应Hench在20世纪60年代发现某些玻璃成分能与活体骨形成较强的化学键结合[25],此后的报道主要对这些材料的生物相容性进行研究[26]。45S5生物活性玻璃(图2)硅含量低(< 60%)、钙/磷比例接近羟基磷灰石,有利于成骨细胞黏附和增殖,使玻璃进入愈合的骨痂中[25]。生物玻璃的存在可加强骨痂对横向运动和剪切力的抵抗,并逐渐溶解,以离子成分排出体外[25]。 研究已发现,少量硅对于生物活性玻璃的功能必不可少,可形成一个更开放的网状结构,这有利于离子与周围介质的相互作用,使其溶解及羟基磷灰石沉淀[27]。少量硅同时降低玻璃的软化温度并增加玻璃的热膨胀,这对于用这些材料作为金属植入物表面的生物活性涂层有不利影响,但可以通过改变玻璃成分的其他方面克服,例如添加K2O、MgO[27]。对生物活性玻璃制造进行的研究,绝大多数文献均关注于改变其组成,以满足医学工程的需要。 生物活性玻璃被称为“A类”生物活性材料,因为它可诱导植入体与宿主组织界面的细胞内和细胞外效应(B类生物活性材料只引发细胞外反应,如组织-材料界面骨生长的骨传导作用)。有研究提出,从玻璃支架植入骨缺损部位开始,生物活性玻璃引发的骨再生涉及12个反应阶段。1-5阶段为植入后24 h内,在交界部位快速释放硅酸等可溶性离子。随着硅酸在植入物表面周围聚集,浓度增加,可聚合成较大的聚合硅酸,最终从溶液中沉淀出来,形成水合硅胶[25]。同时,钙、磷和碳酸根离子从溶液中吸附到植入物表面,形成羟基磷灰石结晶,可促进成骨细胞丝状伪足黏附和成骨细胞增殖,并刺激成骨细胞产生旁分泌生长因子和形态发生蛋白,如骨形态发生蛋白[28]。 研究发现,骨祖细胞在48 h内迁移至生物活性玻璃表面,开始产生Ⅰ型胶原等细胞外基质成分,并迅速矿化,体外培养6-12 d后,成骨细胞成熟形成骨细胞[29]。虽然活体外细胞在初始阶段的生长更慢,但是细胞形态和生长更多表现为成骨细胞表型,表明这些细胞的反应与它们在自然骨修复方式中的反应相似。Xynos等[30]的研究表明,成骨细胞在45S5生物活性玻璃表面生长2 d后,呈现出更紧密堆积状,有很多丝状伪足、背侧隆起和微绒毛,以及细胞激活特征和成骨细胞表型。生长在生物活性玻璃表面的成骨细胞也表现出较多凋亡,培养2 d后,生物活性玻璃表面的成骨细胞比培养在惰性生物材料表面的对照组细胞凋亡通常高5-7倍,反应了自然骨形成中的大量成熟成骨细胞凋亡[31]。 对培养在生物活性玻璃表面的细胞进行分析表明,在细胞周期的DNA合成期(S期)和合成后期(G2-M期)的成骨细胞数量是在生物惰性基体上生长的细胞的2倍[25]。Xynos等[30]通过对1 176个基因的cDNA微阵列分析表明,与对照组相比,45S5生物玻璃溶解的离子产物使60个转录产物水平增加2倍或更多,5个转录产物水平降低。显著上调的基因包括RCL(c-myc激活的生长相关基因)、细胞周期调控因子(如G1/S期特异性细胞周期蛋白D1),以及细胞凋亡的调节器。其他显著上调的基因包括细胞表面黏附分子CD44和整合素β1,以及各种细胞外基质调节因子,包括金属蛋白酶2和金属蛋白酶4及其抑制剂TIMP-1和TIMP-2。与对照组相比,成骨细胞暴露于45S5生物玻璃化学提取物数小时后,胰岛素样生长因子(IGF)轴系统蛋白表达增加300%-500% [32],表明成骨细胞能迅速感受到它们周围环境中钙和/或硅含量的变化。总的来说,溶液中的钙和硅离子被证明能够使200多种基因上调150%以上[30]。 生物玻璃为什么具有生物活性,有理论认为生物材料表面形成的硅酸盐和羟基磷灰石层为生物分子提供了吸附床。伤口周围细胞表达的分子如胰岛素样生长因子1[33],骨形态发生蛋白2,骨形态发生蛋白4和血小板衍生生长因子bb[34],可作为成骨细胞迁移、分化和黏附的趋化信号。有学者试图将这些分子结合在材料表面[35],以提高这些分子在植入物表面的浓度,促进伤口愈合,从而提高植入物的生物活性。Misra等[36]的研究表明,45S5生物玻璃微粒子和纳米粒子能够增加胎牛血清蛋白吸附到聚-3-羟基丁酸酯表面。他们的研究发现,与微粒子相比,20%的生物玻璃纳米粒子可使蛋白吸附增加到30%。 吸附的蛋白活性是其在伤口部位发挥功能的关键,研究表明,吸附到生物玻璃-硅酸盐-羟基磷灰石层表面后,酶活性(如辣根过氧化物酶)持续存在[37]。Manzano等[38]的研究表明,当共价结合到含硅羟基磷灰石表面后,甲状旁腺激素相关蛋白(PTHrP)仍然保持活性。Soderling等[39]的理论认为生物材料的表面能和蛋白质吸附量与其生物相容性成正比。人体内有大量的更大分子阵列,蛋白吸附效应必定更加复杂。 2.5 硅酸盐溶胶-凝胶和多孔硅酸盐纳米粒子 硅比例超过材料的60%时,熔融衍生的生物活性玻璃失去其骨接合能力,研究人员由此得出结论,硅相对于其成骨作用是最重要的。通过沉淀得到完全由硅组成的溶胶-凝胶,可确定玻璃类材料对成骨细胞的影响,而不受生物活性玻璃中其他离子成分的干扰影响。Anderson等[40]发现,与在组织培养板塑料表面的对照组相比,当成骨细胞在硅酸盐表面培养时,成骨细胞黏附于硅胶,而细胞增殖和其他代谢指标保持不变,骨结节(体外骨形成的一个特征)组装增加。李等[41]的研究进一步表明了硅胶表面能诱导磷灰石沉积,液体的pH值、钙/磷比例、镁、氟浓度等因素影响羟基磷灰石沉积的形态。硅胶表面形态对于细胞附着非常重要,除了蛋白吸附,纳米形貌可能影响细胞命运并影响骨样基质形成。溶胶-凝胶工艺过程可被用来制备介孔硅纳米颗粒,这在药物传递和体内成像领域备受关注的[42]。 2.6 多孔硅 多孔硅一般是通过将硅片浸没在氢氟酸电解液中进行阳极处理形成[43]。按照孔径大小分为3种,孔直径小于2 nm的微孔,孔直径在2-50 nm之间的介孔和孔直径大于50 nm的大孔[44]。 许多学者对多孔硅在生物学领域的应用进行了研究,如用于药物控释[45],以及放射性同位素肿瘤内近距离放射治疗不可手术且通常预后较差的肿瘤,如胰腺癌和肝癌[46]。对于肝癌,载有32P小颗粒的多孔硅注入到肿瘤内,可在局部提供非常集中的放射治疗。该技术正在后期(第三期)临床试验中。多孔硅颗粒在体内被降解成硅酸并过肾脏排泄[47]。多孔硅已被证明具有良好的生物相容性[48],可支持多种类型的细胞在体外生长,如成骨细胞[49]、神经元细胞和肝细胞[50-51]。作为胃肠药物载体应用的扩展,多孔硅也可能是一种营养膳食补充剂[52]。 Low等[53]的研究表明,在大鼠眼结膜下植入多孔硅8周后,材料周围出现一层薄的纤维包膜,没有周围组织侵蚀、炎症反应和新生血管形成。Park等[54]也证明多孔硅静脉注射入小鼠后的体内相容性良好:肝脏、肾脏和脾脏内可发现多孔硅颗粒,组织形态正常。注射后1天,在肝脏可发现巨噬细胞,表明小颗粒可被内吞和自然降解,4周后,所有组织都是正常的。 Henstock等[16]采用聚己内酯作为多孔硅微粒的生物可降解载体,发现与含有同样质量45S5生物玻璃的复合材料相比,含有多孔硅的复合材料产生的生物可利用硅酸超过两倍。浸入模拟体液中时,在聚己内酯中加入多孔硅显著增加磷酸钙的沉积。有趣的是,形成的磷灰石钙/磷比例和硅酸浓度成正比,说明含硅羟基磷灰石作为一级反应自发形成。人原代成骨细胞在聚己内酯-多孔硅复合材料表面培养14 d后的碱性磷酸酶活性最高,4周后,成骨细胞增殖及胶原产物与多孔硅含量具有比例关系。J744A.1小鼠巨噬细胞与复合材料共培养结果表明多孔硅不引起免疫反应,甚至可能抑制免疫反应。因此,多孔硅有可能成为骨科组织工程领域具有独特性质的新一代生物材料。 2.7 含硅羟基磷灰石 羟基磷灰石作为体内的骨填充物已得到广泛应用,羟基磷灰石涂层也被涂覆在各种金属关节假体表面以提高骨整合,虽然关于这些涂层效果的临床数据并不确定[55]。体内自发形成的含硅和含碳酸盐羟基磷灰石是生物活性玻璃骨结合能力的一个重要因素,已经有研究者直接将硅掺入到合成羟基磷灰石中,以增强其骨结合性能,生物玻璃溶解产物可重新形成一种矿物质。Bonfield等[56]采用不同的方法如煅烧或磁控共溅射[57],可以在金属(如钛)表面制备单相或薄的含硅羟基磷灰石涂层。 与纯羟基磷灰石相比,这些表面涂层已被证明可显著提高体内的骨整合,这通常是由于这些涂层的更无定形或纳米晶性质,与自然骨矿物质更相似[58]。在模拟体液中,这些涂层表面可形成一层被认为是骨结合必需的含碳酸盐层[59]。在这些涂层材料中,含硅羟基磷灰石可能是研究得最多的,比较符合这些假体生产过程的高温要求。 研究发现,在体外,羟基磷灰石中添加0.8%的硅可增加材料表面的成骨细胞活性,加入2.2%的硅后,成骨细胞活性达到峰值[60]。含硅羟基磷灰石对成骨细胞的影响包括分化加快、碱性磷酸酶表达增加[61],有研究者辩论认为这些影响更多是由于纳米形态引起的物理作用,而不是生物玻璃中发现的复杂的分子反应,因为含硅羟基磷灰石释放到溶液中的硅含量相对较低[55]。 和生物玻璃溶解形成含硅羟基磷灰石一样,提高的生物活性似乎主要来自于羟基磷灰石晶体结构的变化,此时晶胞形态学改变以形成更长、更薄的晶体[56]。很多文献中对变小的含硅羟基磷灰石晶粒大小和溶解度进行了研究[61]。然而,含碳酸盐羟基磷灰石的晶粒大小和溶解度大于含硅羟基磷灰石,但生物活性却低得多[60]。Porter等[62]的研究进一步表明,提高的羟基磷灰石溶解度(始于晶界)对于有效增加羟基磷灰石改建速率从而使其具有生物活性是重要的。 尽管对于含硅羟基磷灰石的生物活性存在不同意见,这些材料在体内的应用研究已取得成功。与未改性羟基磷灰石相比,含硅羟基磷灰石植入绵羊的股骨髁6周后,可导致有序的胶原纤维在材料表面附着速率增加[62]。 2.8 成功应用于临床的含硅生物材料 作为生物材料获得最广泛临床应用的硅化合物无疑是硅酮聚合物[19],几十年来一直被用作导管、引流管、分流器、小关节植入物和整形植入物。有文献认为硅胶乳房假体可引起自身免疫性疾病[63],硅基聚合物潜在的生物降解产物正受到密切关注[64]。 硅基陶瓷如碳化硅也在临床被用作血管支架表面的抗凝血涂层[65]。最近,氮化硅被FDA批准用于脊柱融合[66]和髋关节假体[67]。然而,在所有这些情况下,生物材料被设计为相对生物惰性,但有着极佳的生物耐用性。文章讨论重点为具有生物活性和/或生物可降解的硅基生物材料,有趣的是,硅在生物材料中有各种显著不同的用途,从其惰性和耐用,到其无定形和不稳定,这些取决于元素的化学形态和所使用的制造过程。 "
| [1]Carlisle EM. Silicon: an essential element for the chick. Science. 1972; 178: 619-621.[2]Carlisle EM. Silicon as a trace nutrient. Sci Total Environ. 1988; 73: 95-106.[3]Schwarz K. A bound form of silicon in glycosaminoglycans and polyuronides. Proc Natl Acad Sci. 1973; 70: 1608-1612.[4]Carlisle EM. Silicon as an essential element. Fed Proc. 1974; 33: 1758-1766.[5]Carlisle EM. In vivo requirement for silicon in articular cartilage and connective tissue formation in the chick. J Nutr. 1976; 106: 478-484.[6]Carlisle EM. Silicon. Nutr Rev.1975; 33: 257-261.[7]Landis WJ, Lee DD, Brenna JT, et al. Detection and localization of silicon and associated elements in vertebrate bone tissue by imaging ion microscopy. Calcif Tissue Int.1986; 38: 52-59.[8]Policard A, Collet A, Daniel Moussard H, et al. Deposition of silica in mitochondria: an electron microscopic study.J Biophys Biochem Cytol.1961; 9: 236-238.[9]Arumugam MQ, Ireland DC, Brooks RA,et al. Orthosilicic acid increases collagen type I mRNA expression in human bone-derived osteoblasts in vitro.Key Eng Mater. 2006; 254: 869-872.[10]Reffitt DM, Ogston N, Jugdaohsingh R,et al.Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003; 32: 127-135.[11]Jugdaohsingh R, Reffitt DM, Oldham C,et al. Oligomeric but not monomeric silica prevents aluminum absorption in humans. Am J Clin Nutr.2000; 71: 944-949.[12]Buchman AL, Howard LJ, Guenter P,et al. Micronutrients in parenteral nutrition: too little or too much? The past, present, and recommendations for the future. Gastroenterology. 2009; 137: S1-6.[13]Carlisle EM, Suchil C. Silicon and ascorbate interaction in cartilage formation in culture. Fed Proc.1983; 42: 398.[14]Lia H,Changa J.Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater.2013; 9: 6981-6991.[15]Bouletreau PJ, Warren SM, Spector JA,et al.Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg.2002; 109: 2384-2397.[16]Henstock JR, Ruktanonchai UR, Canham LT,et al. Porous silicon confers bioactivity to polycaprolactone composites in vitro.J Mater Sci Mater Med.2014; 25: 1087-1097.[17]Scarfi S, Benatti U, Pozzolini M,et al. Ascorbic acid-pretreated quartz enhances cyclo-oxygenase-2 expression in RAW 264.7 murine macrophages. FEBS J. 2007; 274: 60-73.[18]Landis WJ, Glimcher MJ. Electron diffractiona and electron probe microanalysis of the mineral phase of bone tissue prepared by anhydrous techniques. J Ultrastruct Res.1978; 63: 188-223.[19]Ratner BD, Hoffman AS, Schoen FJ,et al. Biomaterials science: an introduction to materials in medicine. New York: Academic Press; 2004.[20]Boivin G. The hydroxyapatite crystal: a closer look. Medicographia. 2007; 29: 126-132.[21]Shepherd JH, Shepherd DV, Best SM. Substituted hydroxyapatites for bone repair. J Mater Sci Mater Med. 2012; 23: 2335-2347.[22]Jugdaohsingh R, Tucker KL, Qiao N,et al. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort.J Bone Min Res.2004; 19: 297-307.[23]Birchall JD. The essentiality of silicon in biology. Chem Soc Rev. 1995; 24: 351-357.[24]Elliot MA, Edwards HM. Effect of dietary silicon on growth and skeletal development in chickens. J Nutr. 1991; 121: 201-207.[25]Hench LL. The story of Bioglass. J Mater Sci-Mater Med. 2006; 17: 967-978.[26]Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater 2013; 9: 4457-486.[27]Lopez-Esteban A, Saiz E, Fujino S,et al. Bioactive glass coatings for orthopaedic metallic implants.J Eur Ceram Soc. 2003; 23: 2921-2930.[28]Thomas MV, Puleo DA, Al-Sabbagh M. Bioactive glass three decades on. J Long Term Eff Med Implants. 2005; 15: 585-597.[29]Loty C, Sautier J-M, Boulekbache H,et al. In vitro bone formation on a bone-like apatite layer prepares by a biomimetic process on a bioactive glass-ceramic. J Biomed Mater Res.1999; 49: 423-434.[30]Xynos IE, Alasdair JE, Buttery LDK,et al. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5. J Biomed Mater Res. 2001; 55: 151-157.[31]Gough JE, Downes S. Osteoblast cell death on methacrylate polymers involves apoptosis.J Biomed Mater Res, Part A. 2001;15: 497-505.[32]Valerio P, Pereira MM, Goes AM,et al. The effect of ionic products from bioactive glass dissolution on osteoblast proliferation and collagen production. Biomaterials. 2004; 25: 2941-2948.[33]Nakasaki M, Yoshioka K, Miyamoto Y,et al. IGF-I secreted by osteoblasts acts as a potent chemotactic factor for osteoblasts. Bone. 2008; 43: 869-879.[34]Fiedler J, Roderer G, Gunther KP,et al. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002; 87: 305-312.[35]Rosengren A, Oscarsson S, Mazzocchi M,et al. Protein adsorption onto two bioactive glass-ceramics. Biomaterials. 2003; 24: 147-155.[36]Misra SK, Mohn D, Brunner TJ,et al. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composites. Biomaterials 2008;29: 1750-1761.[37]Lobell KD, Hench LL. In vitro adsorption and activity of enzymes on reaction layers of bioactive glass substrates. J Biomed Mater Res, Part A.1998; 39: 575-579.[38]Manzano M, Lozano D, Arcos D,et al.Comparison of the osteoblastic activity conferred on Si-doped hydroxyapatite scaffolds by different osteostatin coatings. Acta Biomater.2011; 7: 3555-3562.[39]Soderling E, Herbst K, Larmas E,et al. Protein adsorption to a bioactive glass with special reference to precorrosion. J Biomed Mater Res, Part A.1996; 31: 525-531.[40]Anderson SI, Downes S, Perry CC,et al. Evaluation of the osteoblast response to a silica gel in vitro. J Mater Sci Mater Med. 1998; 9: 731-735.[41]Li P, Nakanishi K, Kokubo T,et al. Induction and morphology of hydroxyapatite, precipitated from metastable simulated body fluids on sol-gel prepared silica. Biomaterials. 1993; 14: 963-968.[42]Rosenholm JM, Mamaeva V, Sahlgren C, et al. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine. 2012; 7(1): 111-120.[43]Seo YH, Nahm KS, An MH, et al. Formation mechanism and pore size control of light-emitting porous silicon.J Appl Phys. 1994; 33: 6425-6431.[44]Canham LT. Properties of porous silicon. London: Inspec, IEE; 1997.[45]Serda RE, Gu J, Bhavane RC, Liu X, et al. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009; 30: 2440-2448.[46]Zhang K, Loong SL, Connor S, et al. Complete tumor response following intratumoral 32P BioSilicon on human hepatocellular and pancreatic carcinoma xenografts in nude mice. Clin Cancer Res. 2005; 11: 7532-7537.[47]Canham LT, Aston R. Will a chip every day keep the doctor away?. Phys World. 2001: 27-31.[48]Jaganathan H, Godin B. Biocompatibility assessment of Si-based nano- and micro-particles. Adv Drug Deliv Rev.2012; 64(15): 1800-1819.[49]Whitehead MA, Fan D, Mukherjee P,et al. Highporosity poly(epsilon-caprolactone)/mesoporous silicon scaffolds: calcium phosphate deposition and biological response to bone precursor cells. Tissue Eng Part A. 2008; 14: 195-206.[50]Johansson F, Kanje M, Linsmeier CE,et al. The influence of porous ilicon on axonal outgrowth in vitro. IEEE Trans Biomed Eng.2008; 55: 1447-1449. [51]Alvarez SD, Derfus AM, Schwartz MP,et al. The compatibility of hepatocytes with chemically modified porous silicon with reference to in vitro biosensors. Biomaterials. 2008; 30: 26-34.[52]Shabir Q, Skaria C, O’Brien H,et al. Taste and mouthfeel assessment of porous and non-porous silicon microparticles. Nanoscale Res Lett.2012; 7: 407.[53]Low SP, Voelcker NH, Canham LT,et al. The biocompatibility of porous silicon in tissues of the eye. Biomaterials. 2009; 30: 2873-2880.[54]Park JH, Gu L, von Maltzahn G,et al. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater.2009; 8: 331-336.[55]Bohner M. Silicon-substituted calcium phosphates–a critical view. Biomaterials. 2009; 30: 6403-6406.[56]Gibson IR, Best SM, Bonfield W. Chemical characterization of siliconsubstituted hydroxyapatite. J Biomed Mater Res.1999; 44: 422-428.[57]Thian ES, Huang J, Best SM,et al. Silicon-substituted hydroxyapatite: The next generation of bioactive coatings. Mater Sci Eng, C. 2007; 27: 251-256.[58]Zhang E, Zou C. Porous titanium and silicon-substituted hydroxyapatite biomodification prepared by a biomimetic process: characterization and in vivo evaluation. Acta Biomater. 2009; 5: 1732-1741.[59]Botelho CM, Brooks RA, Best SM,et al. Human osteoblast response to silicon-substituted hydroxyapatite.J Biomed Mater Res,Part A.2006; 79: 723-730.[60]Porter AE, Patel N, Skepper JN, et al. Effect of sintered silicatesubstituted hydroxyapatite on remodelling processes at the bone-implant interface. Biomaterials. 2004; 25: 3303-3314.[61]Gibson IR, Best SM, Bonfield W. Effect of silicon substitution on the sintering and microstructure of hydroxyapatite. J Am Ceram Soc.2002; 85: 2771-2777.[62]Porter AE, Patel N, Skepper JN, et al. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials. 2003; 24: 4609-4620.[63]Janowsky EC, Kupper LL, Hulka BS. Meta-analysis of the relation between silicon breast implants and the risk of connected tissue diseases. N Eng J Med. 2000; 342(11): 781-790.[64]Lugowksi SJ, Smith DC, Bonek H,et al. Analysis of silicon in human tissues with special reference to silicone breast implants. J Trace Elem Med Biol.2000; 14: 31-42.[65]Heublein B, Ozbek C, Pethig K. Silicon carbide-coated stents: clinical experience in coronary lesions with increased thrombotic risk. J Endovasc Surg. 1998; 5: 32-36.[66]Webster TJ, Patel AA, Rahaman MN, et al. Anti infective and osteointegration properties of silicon nitride, poly(ether ether ketone) and titanium implants. Acta Biomater. 2012; 8(12): 4447-4454.[67]Olofsson J, Grehk TM, Berlind T,et al. Evaluation of silicon nitride as a wear resistant and resorbable alternative for total hip joint replacement. Biomatter. 2012; 2(2): 1-9.[68]Thomas MV, Puleo DA, Al-Sabbagh, M. Bioactive glass three decades on. J Long Term Eff Med Implants. 2005; 15: 585-597. |
| [1] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [2] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1530-1536. |
| [3] | Hou Jianfei, Wang Fuke, Yang Guiran, Liao Xinyu, Jia Di, Liu Dejian. Application advantages of hydroxyapatite surface modification as a bone scaffold for tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1610-1614. |
| [4] | Liu Jiangfeng. Nano-hydroxyapatite/polyamide 66 composite filling combined with locking plate in the treatment of fibrous dysplasia of femoral bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 542-547. |
| [5] | Ma Zhijie, Li Jingyu, Cao Fang, Liu Rong, Zhao Dewei. Influencing factors and biological property of novel biomedical materials: porous silicon carbide coated with bioactive tantalum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 558-563. |
| [6] | Liu Changhao, Zheng Jianping, Shi Jiandang, Zhu Xi, Zhou Zhanwen, Zhang Xu. Three-dimensional printed artificial bone loaded with anti-tuberculosis drugs and bone morphogenetic protein 2 sustained-release microspheres can promote osteogenesis of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(28): 4447-4453. |
| [7] | Li Rui. Biological characteristics of hydroxyapatite/chitosan combined with metformin for bone defect in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(28): 4460-4464. |
| [8] | Sun Yang, Luo Mingran, Zheng Li, Hu Weifan, Yuan Feng. Influence of plasma spraying and electrochemical deposition of hydroxyapatite coating morphology on bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(28): 4516-4522. |
| [9] | Liao Xinyu, Wang Fuke, Wang Guoliang. Progress and challenges of bone tissue engineering scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(28): 4553-4560. |
| [10] | He Lin, Wu Xi, He Song, Yang Sen. Hydrophilicity and cell adhesion of hydroxyapatite bioceramics after the coating of polydopamine [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3540-3544. |
| [11] | Zhang Xianjun, Zhao Xijiang. In vivo osteogenic properties of silicon-incorporated titanium dioxide nanotubes on titanium screw surface [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2461-2465. |
| [12] | Wang Renxian, Cao Jingjing, Wang Honggang, Wan Ben, Liu Weifeng. Effects of dispersants on aggregation, intracellular distribution and cell proliferation of nano-hydroxyapatite [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2500-2505. |
| [13] | Wang Hongyuan, Wang Wei, Yang Shuqing, Dou Lixin, Liu Lijun. Preparation and properties of porous nitrogen oxygen bioglass scaffold for bone repair [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2521-2527. |
| [14] | Zhang Lixing, Tian Ang, Li Xi, Bai Xizhuang. Drug-release characteristic and biological toxicity of TiO2 nanotube/hydroxyapatite loaded vancomycin coating [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1500-1506. |
| [15] | Wang Qian, Li Lu, Shu Jingyuan, Dong Zhiheng, Jin Youshi, Wang Qingshan. Micro-morphology and phase of zirconia-based nano-hydroxyapatite functional gradient biomaterials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1517-1521. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||