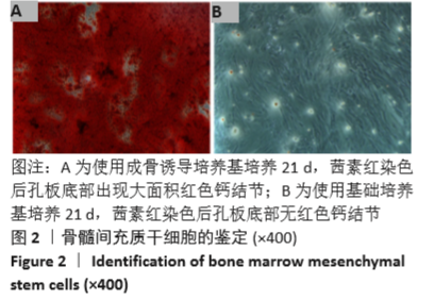
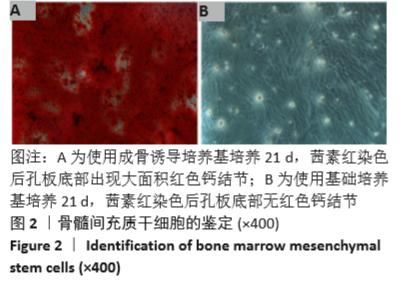
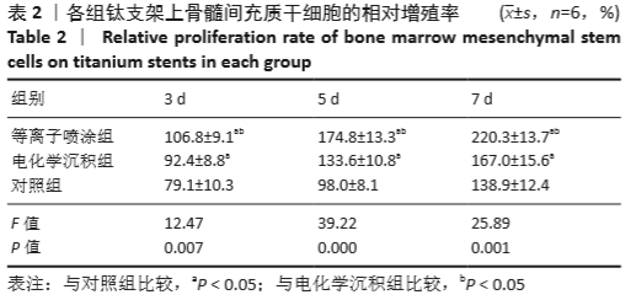
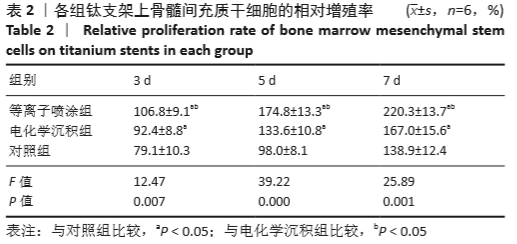
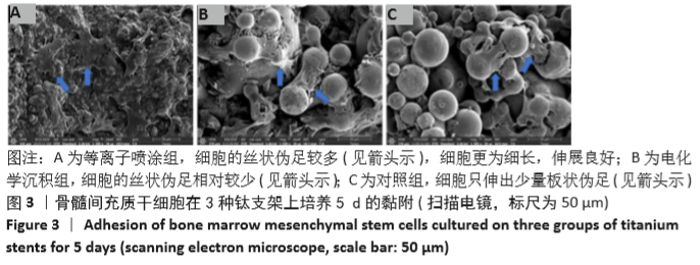
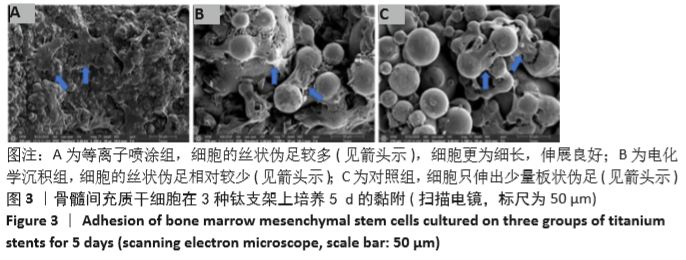
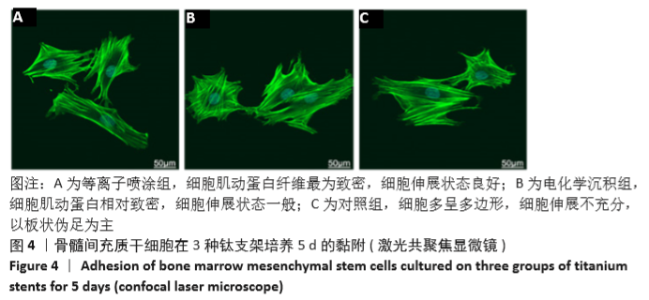
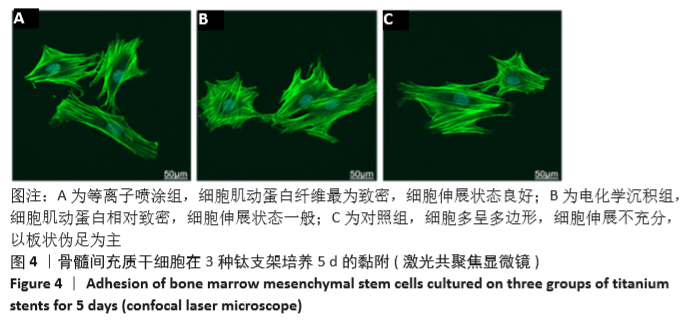
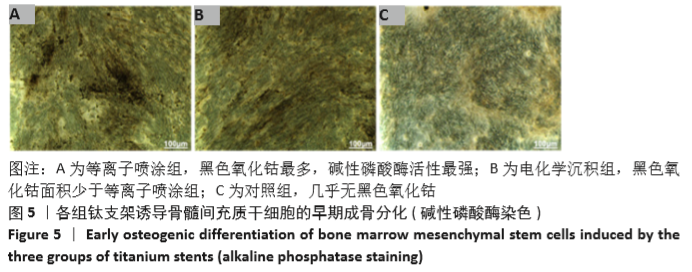
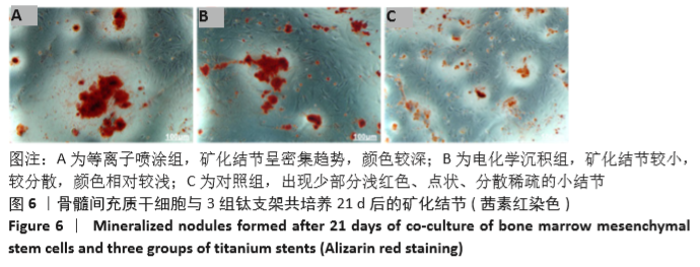
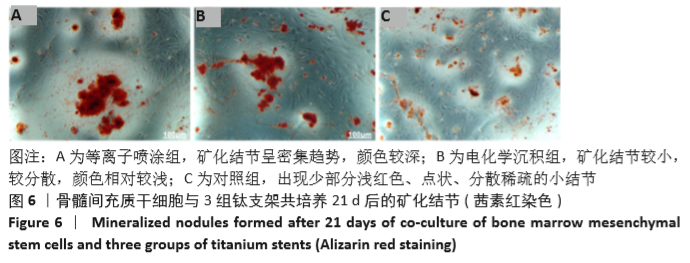
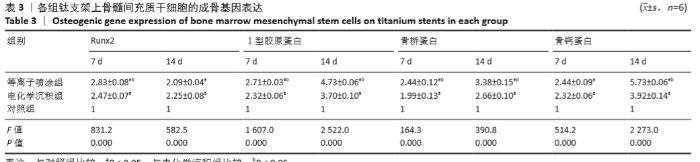
Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (28): 4516-4522.doi: 10.12307/2021.067
Previous Articles Next Articles
Influence of plasma spraying and electrochemical deposition of hydroxyapatite coating morphology on bone marrow mesenchymal stem cells
Sun Yang1, Luo Mingran1, Zheng Li1, Hu Weifan1, Yuan Feng2
- 1Graduate School of Xuzhou Medical University, Xuzhou 221000, Jiangsu Province, China; 2Department of Orthopedics, Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu Province, China
-
Received:2020-10-15Revised:2020-10-17Accepted:2020-11-13Online:2021-10-08Published:2021-05-21 -
Contact:Yuan Feng, Professor, Department of Orthopedics, Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu Province, China -
About author:Sun Yang, Master candidate, Physician, Graduate School of Xuzhou Medical University, Xuzhou 221000, Jiangsu Province, China -
Supported by:the Natural Science Foundation of Jiangsu Province, No. BE2016647 (to YF)
CLC Number:
Cite this article
Sun Yang, Luo Mingran, Zheng Li, Hu Weifan, Yuan Feng. Influence of plasma spraying and electrochemical deposition of hydroxyapatite coating morphology on bone marrow mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(28): 4516-4522.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] AHMADI SM, HEDAYATI R, LI Y, et al. Fatigue performance of additively manufactured meta-biomaterials: the effects of topology and material type. Acta Biomater. 2018;65:292-304. [2] GADIA A, SHAH K, NENE A. Emergence of Three-Dimensional Printing Technology and Its Utility in Spine Surgery. Asian Spine J. 2018;12(2): 365-371. [3] TRAUNER KB. The Emerging Role of 3D Printing in Arthroplasty and Orthopedics. J Arthroplasty. 2018;33(8):2352-2354. [4] PATI F, SONG T H, RIJAL G, et al. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 2015;37:230-241. [5] LIU A, XUE GH, SUN M, et al. 3D printing surgical implants at the clinic: A experimental study on anterior cruciate ligament reconstruction. Sci Rep. 2016;6:21704. [6] SILVIA S, SEIJI Y, FRANCESCO B, et al. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2019;83:37-54. [7] LEUKERS B, GÜLKAN H, IRSEN SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing.J Mater Sci Mater Med. 2005;16(12):1121-1124. [8] RINCÓN-LÓPEZ JA, HERMANN-MUÑOZ JA, GIRALDO-BETANCUR AL, et al. Synthesis, Characterization and In Vitro Study of Synthetic and Bovine-Derived Hydroxyapatite Ceramics: A Comparison. Materials (Basel). 2018;11(3):333. [9] ZHU J, SUN HH, WO J, et al. Duration of electrochemical deposition affects the morphology of hydroxyapatite coatings on 3D-printed titanium scaffold as well as the functions of adhered MC3T3-E1 cells. J Orthop Sci. 2020;25(4):708-714. [10] VILARDELL AM, CINCA N, GARCIA-GIRALT N, et al. In-vitro comparison of hydroxyapatite coatings obtained by cold spray and conventional thermal spray technologies. Mater Sci Eng C Mater Biol Appl. 2020; 107:110306. [11] XUEREB M, CAMILLERI J, ATTARD N. Systematic Review of Current Dental Implant Coating Materials and Novel Coating Techniques. Int J Prosthodont. 2015;28(1):51-59. [12] 李成龙,李文戈,赵远涛,等.降低等离子喷涂涂层孔隙率的研究进展[J].机械工程材料,2020,44(5):60-65. [13] 杨学兵,张林伟.水蒸气处理对钛合金表面羟基磷灰石涂层结构的影响[J].表面技术,2020,49(9):167-174,205. [14] FATHYUNES L, KHALIL-ALLAFI J. Effect of employing ultrasonic waves during pulse electrochemical deposition on the characteristics and biocompatibility of calcium phosphate coatings. Ultrason Sonochem. 2018;42:293-302. [15] 王天云,张青,朱良均,等.利用电化学法结合丝素膜调控羟基磷灰石沉积及其形貌[J].浙江大学学报(农业与生命科学版),2018, 44(2):209-214. [16] ALBAYRAK O, EL-ATWANI O, ALTINTAS S. Hydroxyapatite coating on titanium substrate by electrophoretic deposition method: Effects of titanium dioxide inner layer on adhesion strength and hydroxyapatite decomposition. Surf Coat Technol. 2008;202(11):2482-2487. [17] KINNAIRD T, STABILE E, BURNETT MS, et al. Marrow-Derived Stromal Cells Express Genes Encoding a Broad Spectrum of Arteriogenic Cytokines and Promote In Vitro and In Vivo Arteriogenesis Through Paracrine Mechanisms. Circ Res. 2004;94(5):678-685. [18] AMNA T. Valorization of Bone Waste of Saudi Arabia by Synthesizing Hydroxyapatite. Appl Biochem Biotechnol. 2018;186:779-788. [19] LIN K, WU C, CHANG J, et al. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014;10(10):4071-102. [20] EPPLE M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018;77:1-14. [21] SHUMIN P, YUAN H, PING H, et al. Fabrication of Two Distinct Hydroxyapatite Coatings and Their Effects on MC3T3-E1 Cell Behavior. Colloids Surf B Biointerfaces. 2018;171:40-48. [22] KUBOKI Y, JIN Q, TAKITA H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 2):S105-115. [23] HAYAKAWA T, KAWASHITA M, TAKAOAKA GH. Coating of hydroxyapatite films on titanium substrates by electrodeposition under pulse current. J Ceram Soc Jpn. 2008;116(1349):68-73. [24] HULBERT SF, YOUNG FA, MATHEWS RS, et al. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res. 1970;4(3):433-456. [25] MASAHIRO N, MASAHIRO Y, MASATO W, et al. Activation of Osteoblastic Function on Titanium Surface with Titanium-Doped Hydroxyapatite Nanoparticle Coating: An In Vitro Study. Int J Oral Maxillofac Implants. 2017;32(4):779-791. [26] WANG J, WANG M, CHEN F, et al. Nano-Hydroxyapatite Coating Promotes Porous Calcium Phosphate Ceramic-Induced Osteogenesis Via BMP/Smad Signaling Pathway. Int J Nanomedicine. 2019;14: 7987-8000. [27] HAMIDABADI HG, SHAFAROUDI MM, SEIFI M, et al. Repair of Critical-Sized Rat Calvarial Defects With Three-Dimensional Hydroxyapatite-Gelatin Scaffolds and Bone Marrow Stromal Stem Cells. Med Arch. 2018;72(2):88-93. [28] ROYCROFT A, MAYOR R. Michael Abercrombie: contact inhibition of locomotion and more. Int J Dev Biol. 2018;62(1-2-3):5-13. [29] SIPAHI R, ZUPANC GKH. Stochastic cellular automata model of neurosphere growth: Roles of proliferative potential, contact inhibition, cell death, and phagocytosis. J Theor Biol. 2018;445:151-165. [30] CUI JG, CHEN G, PERRY AS, et al. Transient Cell-to-Cell Signaling before Mitosis in Cultures of human Bone Marrow-Derived Mesenchymal Stem/Stromal Cells. Stem Cells Dev. 2019;28(2):120-128. [31] PAPAGEORGIOU P, VALLMAJO-MARTIN Q, KISIELOW M, et al. Expanded skeletal stem and progenitor cells promote and participate in induced bone regeneration at subcritical BMP-2 dose. Biomaterials. 2019;217: 119278. [32] LI X, ZOU Q, LI W, et al. Intracellular Interaction of Hydroxyapatite-Based Nanocrystals with Uniform Shape and Traceable Fluorescence. Inorg Chem. 2018;57(21):13739-13748. [33] PEI YF, LIU L, LIU TL, et al. Joint Association Analysis Identified 18 New Loci for Bone Mineral Density. J Bone Miner Res. 2019;34(6): 1086-1094. [34] LIN Z, HE H, WANG M, et al. MicroRNA-130a controls bone marrow mesenchymal stem cell differentiation towards the osteoblastic and adipogenic fate. Cell Prolif. 2019;52:e12688. |
| [1] | Du Xiupeng, Yang Zhaohui. Effect of degree of initial deformity of impacted femoral neck fractures under 65 years of age on femoral neck shortening [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1410-1416. |
| [2] | Zhang Shangpu, Ju Xiaodong, Song Hengyi, Dong Zhi, Wang Chen, Sun Guodong. Arthroscopic suture bridge technique with suture anchor in the treatment of acromioclavicular dislocation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1417-1422. |
| [3] | Liang Yan, Zhao Yongfei, Xu Shuai, Zhu Zhenqi, Wang Kaifeng, Liu Haiying, Mao Keya. Imaging evaluation of short-segment fixation and fusion for degenerative lumbar scoliosis assisted by highly selective nerve root block [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1423-1427. |
| [4] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [5] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [6] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [7] | Chen Jinping, Li Kui, Chen Qian, Guo Haoran, Zhang Yingbo, Wei Peng. Meta-analysis of the efficacy and safety of tranexamic acid in open spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1458-1464. |
| [8] | Hu Kai, Qiao Xiaohong, Zhang Yonghong, Wang Dong, Qin Sihe. Treatment of displaced intra-articular calcaneal fractures with cannulated screws and plates: a meta-analysis of 15 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1465-1470. |
| [9] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [10] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [11] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [12] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [13] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [14] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [15] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||