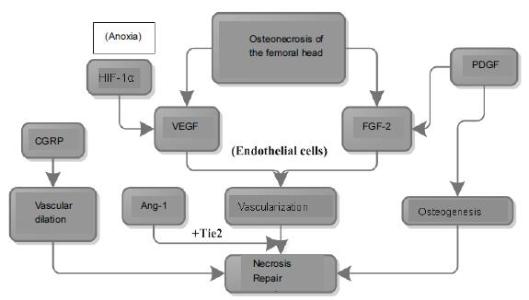
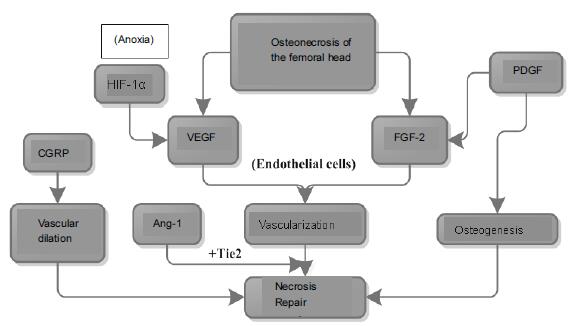
Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (15): 2197-2205.doi: 10.3969/j.issn.2095-4344.2016.15.010
Previous Articles Next Articles
Angiogenic factors in osteonecrosis of the femoral head
Hong Guo-ju1, He Wei2, Wei Qiu-shi2, Chen Lei-lei2
- 1 Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong Province, China
2 First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510407, Guangdong Province, China
-
Received:2016-02-01Online:2016-04-08Published:2016-04-08 -
Contact:Chen Lei-lei, M.D., Attending physician, First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510407, Guangdong Province, China -
About author:Hong Guo-ju, Studying for master’s degree, Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong Province, China -
Supported by:the National Nature Science Foundation of China, No. 81473697
Cite this article
Hong Guo-ju, He Wei, Wei Qiu-shi, Chen Lei-lei. Angiogenic factors in osteonecrosis of the femoral head[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(15): 2197-2205.
share this article
| [1] Seamon J, Keller T, Saleh J, et al. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:601763. [2] Riddle RC, Khatri R, Schipani E, et al. Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. J Mol Med (Berl). 2009;87(6):583-590. [3] Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14(16):1983-1991. [4] Wenger RH, Rolfs A, Spielmann P, et al. Mouse hypoxia-inducible factor-1alpha is encoded by two different mRNA isoforms: expression from a tissue-specific and a housekeeping-type promoter. Blood. 1998;91(9):3471-3480. [5] Wan C, Shao J, Gilbert SR, et al. Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci. 2010;1192:322-326. [6] Shomento SH, Wan C, Cao X, et al. Hypoxia-inducible factors 1alpha and 2alpha exert both distinct and overlapping functions in long bone development. J Cell Biochem. 2010;109(1):196-204. [7] Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437-443. [8] Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225(2): 485-488. [9] Li J, Fan L, Yu Z, et al. The effect of deferoxamine on angiogenesis and bone repair in steroid-induced osteonecrosis of rabbit femoral heads. Exp Biol Med (Maywood). 2015;240(2):273-280. [10] Radke S, Battmann A, Jatzke S, et al. Expression of the angiomatrix and angiogenic proteins CYR61, CTGF, and VEGF in osteonecrosis of the femoral head. J Orthop Res. 2006;24(5):945-952. [11] Mazumdar J, Dondeti V, Simon MC. Hypoxia-inducible factors in stem cells and cancer. J Cell Mol Med. 2009;13(11-12):4319-4328. [12] Manalo DJ, Rowan A, Lavoie T, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105(2):659-669. [13] Ding H, Gao YS, Hu C, et al. HIF-1α transgenic bone marrow cells can promote tissue repair in cases of corticosteroid-induced osteonecrosis of the femoral head in rabbits. PLoS One. 2013;8(5):e63628. [14] Hong JM, Kim TH, Chae SC, et al. Association study of hypoxia inducible factor 1alpha (HIF1alpha) with osteonecrosis of femoral head in a Korean population. Osteoarthritis Cartilage. 2007;15(6):688-694. [15] Chachami G, Kalousi A, Papatheodorou L, et al. An association study between hypoxia inducible factor-1alpha (HIF-1α) polymorphisms and osteonecrosis. PLoS One. 2013;8(11):e79647. [16] Hoeben A, Landuyt B, Highley MS, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549-580. [17] Whitlock PR, Hackett NR, Leopold PL, et al. Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Mol Ther. 2004;9(1):67-75. [18] Varoga D, Drescher W, Pufe M, et al. Differential expression of vascular endothelial growth factor in glucocorticoid-related osteonecrosis of the femoral head. Clin Orthop Relat Res. 2009;467(12):3273-3282. [19] Suzuki O, Bishop AT, Sunagawa T, et al. VEGF-promoted surgical angiogenesis in necrotic bone. Microsurgery. 2004;24(1):85-91. [20] Bouletreau PJ, Warren SM, Spector JA, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109(7):2384-2397. [21] Wang W, Li S, Niu D. Study on relationship between osteoporosis and mRNA expressions of vascular endothelial growth factor and bone morphogenetic protein 2 in nontraumatic avascular necrosis of femoral head. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24(9):1072-1077. [22] Yang C, Yang SH, Du JY, et al. Basic fibroblast growth factor gene transfection to enhance the repair of avascular necrosis of the femoral head. Chin Med Sci J. 2004;19(2):111-115. [23] Jia Y, Chu TW, Zhou Y, Luo G, et al. Enhancing blood flow of necrotic femoral head by adenovirus hu-VEGF121 transfection in rabbits. Disan Junyi Daxue Xuebao. 2005;27(9):885-887. [24] Kim T, Hong JM, Lee J, et al. Promoter polymorphisms of the vascular endothelial growth factor gene is associated with an osteonecrosis of the femoral head in the Korean population. Osteoarthritis Cartilage. 2008; 16(3):287-291. [25] Lee YJ, Lee JS, Kang EH, et al. Vascular endothelial growth factor polymorphisms in patients with steroid-induced femoral head osteonecrosis. J Orthop Res. 2012;30(1):21-27. [26] Liu B, Cao Y, Wang D, et al. Vascular endothelial growth factor -634G/C polymorphism associated with osteonecrosis of the femoral head in a Chinese population. Genet Test Mol Biomarkers. 2012;16(7):739-743. [27] Hong JM, Kim TH, Kim HJ, et al. Genetic association of angiogenesis- and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp Mol Med. 2010;42(5):376-385. [28] Egger M, Schgoer W, Beer AG, et al. Hypoxia up-regulates the angiogenic cytokine secretoneurin via an HIF-1alpha- and basic FGF-dependent pathway in muscle cells. FASEB J. 2007;21(11):2906-2917. [29] Wang W, Li S, Niu D, et al. Relationship between the bone mass and the expressions of vascular endothelial growth factor, basic fibroblast growth factor, and bone morphogenetic protein 2 mRNA in avascular necrosis of femoral head. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25(8):984-991. [30] Li W, Sakai T, Nishii T, et al. Distribution of TRAP-positive cells and expression of HIF-1alpha, VEGF, and FGF-2 in the reparative reaction in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27(5):694-700. [31] Ingber D. Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J Cell Biochem. 1991;47(3):236-241. [32] Moscatelli D, Presta M, Rifkin DB. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci USA. 1986;83(7): 2091-2095. [33] Nakamae A, Sunagawa T, Ishida O, et al. Acceleration of surgical angiogenesis in necrotic bone with a single injection of fibroblast growth factor-2 (FGF-2). J Orthop Res. 2004;22(3):509-513. [34] Eppley BL, Connolly DT, Winkelmann T, et al. Free bone graft reconstruction of irradiated facial tissue: experimental effects of basic fibroblast growth factor stimulation. Plast Reconstr Surg. 1991;88(1):1-11. [35] Globus RK, Patterson-Buckendahl P, Gospodarowicz D. Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor beta. Endocrinology. 1988;123(1):98-105. [36] Li X, Gong Y, Song Y, et al. Study on the effect of composite of basic fibroblast growth factor and partially deproteinized bone on the repair of femoral head defects. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(3):183-186. [37] Kuroda Y, Akiyama H, Kawanabe K, et al. Treatment of experimental osteonecrosis of the hip in adult rabbits with a single local injection of recombinant human FGF-2 microspheres. J Bone Miner Metab. 2010;28(6): 608-616. [38] Yang C, Yang SH, Du JY, et al. Basic fibroblast growth factor gene transfection to enhance the repair of avascular necrosis of the femoral head. Chin Med Sci J. 2004;19(2):111-115. [39] Shah P, Keppler L, Rutkowski J. A review of platelet derived growth factor playing pivotal role in bone regeneration. J Oral Implantol. 2014;40(3):330-340. [40] Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006;81(9):1241-1257. [41] Chung R, Foster BK, Zannettino AC, et al. Potential roles of growth factor PDGF-BB in the bony repair of injured growth plate. Bone. 2009;44(5):878-885. [42] Grotendorst GR, Pencev D, Martin GR, et al. Molecular mediators of tissue repair. In: Hunt TK, Heppenstall RB, Pines E, Rovee D, eds. Soft and Hard Tissue Repair. New York: Praeger, 1984:20-40. [43] Grotendorst GR, Martin GR, Pencev D, et al. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985;76(6):2323-2329. [44] Lindahl P, Johansson BR, Levéen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323): 242-245. [45] Cao R, Bråkenhielm E, Pawliuk R, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9(5):604-613. [46] Yang M, Khachigian LM, Hicks C, et al. Identification of PDGF receptors on human megakaryocytes and megakaryocytic cell lines. Thromb Haemost. 1997; 78(2):892-896. [47] Pósán E, Szepesi K, Gáspár L, et al. Thrombotic and fibrinolytic alterations in the aseptic necrosis of femoral head. Blood Coagul Fibrinolysis. 2003;14(3): 243-248. [48] Cenni E, Fotia C, Rustemi E, et al. Idiopathic and secondary osteonecrosis of the femoral head show different thrombophilic changes and normal or higher levels of platelet growth factors. Acta Orthop. 2011; 82(1):42-49. [49] Lerner UH. Deletions of genes encoding calcitonin/alpha-CGRP, amylin and calcitonin receptor have given new and unexpected insights into the function of calcitonin receptors and calcitonin receptor-like receptors in bone. J Musculoskelet Neuronal Interact. 2006;6(1):87-95. [50] Fernandez S, Knopf MA, Bjork SK, et al. Bone marrow-derived macrophages express functional CGRP receptors and respond to CGRP by increasing transcription of c-fos and IL-6 mRNA. Cell Immunol. 2001;209(2):140-148. [51] Hoff AO, Catala-Lehnen P, Thomas PM, et al. Increased bone mass is an unexpected phenotype associated with deletion of the calcitonin gene. J Clin Invest. 2002;110(12):1849-1857. [52] Owan I, Ibaraki K. The role of calcitonin gene-related peptide (CGRP) in macrophages: the presence of functional receptors and effects on proliferation and differentiation into osteoclast-like cells. Bone Miner. 1994;24(2):151-164. [53] Gibbins IL, Furness JB, Costa M, et al. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett. 1985;57(2):125-130. [54] Cao T, Pintér E, Al-Rashed S, et al. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J Immunol. 2000;164(10): 5424-5429. [55] Wang L, Wang K, Zhang L, et al. Study on effect of sensory neuropeptide in steroid-induced avascular necrosis of femoral head. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24(9):1078-1081. [56] Wang L, Wang N, Li M, et al. To investigate the role of the nervous system of bone in steroid-induced osteonecrosis in rabbits. Osteoporos Int. 2010;21(12): 2057-2066. [57] Li J, Wang Y, Li Y, et al. The effect of combined regulation of the expression of peroxisome proliferator-activated receptor-γ and calcitonin gene-related peptide on alcohol-induced adipogenic differentiation of bone marrow mesenchymal stem cells. Mol Cell Biochem. 2014;392(1-2):39-48. [58] Wang YS, Wang YH, Zhao GQ, et al. Osteogenic potential of human calcitonin gene-related peptide alpha gene-modified bone marrow mesenchymal stem cells. Chin Med J (Engl). 2011;124(23):3976-3981. [59] Toda M, Suzuki T, Hosono K, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008;62(6):352-359. [60] Mishima T, Ito Y, Hosono K, et al. Calcitonin gene-related peptide facilitates revascularization during hindlimb ischemia in mice. Am J Physiol Heart Circ Physiol. 2011; 300(2):H431-439. [61] Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7): 1161-1169. [62] Schnürch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119(3):957-968. [63] Augustin HG, Koh GY, Thurston G, et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009; 10(3): 165-177. [64] Jeansson M, Gawlik A, Anderson G, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011;121(6):2278-2289. [65] Homer JJ, Greenman J, Drevs J, et al. Soluble Tie-2 receptor levels independently predict locoregional recurrence in head and neck squamous cell carcinoma. Head Neck. 2002;24(8):773-778. [66] Makinde T, Agrawal DK. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J Cell Mol Med. 2008;12(3):810-828. [67] Thurston G. Complementary actions of VEGF and angiopoietin-1 on blood vessel growth and leakage. J Anat. 2002;200(6):575-580. Park BH, Jang KY, Kim KH, et al. COMP-Angiopoietin-1 ameliorates surgery-induced ischemic necrosis of the femoral head in rats. Bone. 2009;44(5):886-892. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| [5] | Ma Zhijie, Li Jingyu, Cao Fang, Liu Rong, Zhao Dewei. Influencing factors and biological property of novel biomedical materials: porous silicon carbide coated with bioactive tantalum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 558-563. |
| [6] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [7] | Shi Xiaoxiu, Mao Shilong, Liu Yang, Ma Xingshuang, Luo Yanfeng. Comparison of tantalum and titanium (alloy) as orthopedic materials: physical and chemical indexes, antibacterial and osteogenic ability [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 593-599. |
| [8] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [9] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [10] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [11] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [12] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [13] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [14] | Yang Junhui, Luo Jinli, Yuan Xiaoping. Effects of human growth hormone on proliferation and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3956-3961. |
| [15] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||