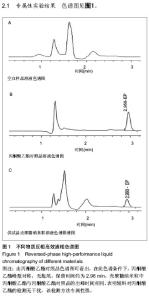
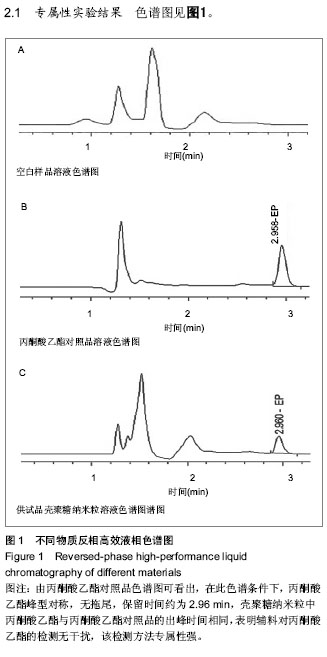
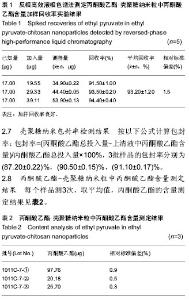
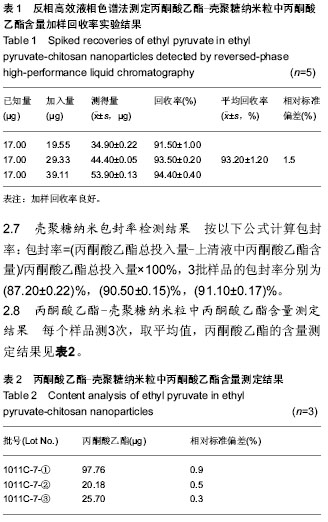
| [1] 乔京贵,赵中夫.丙酮酸乙酯的抗炎作用研究进展[J].长治医学院学报,2005,19(4):318-320.
[2] Yang R,Gallo DJ,Baust JJ,et al.Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock.Am J Physiol Gastrointest Liver Physiol. 2002;283:G212-221.
[3] Kao KK,Fink MP.The biochemical basis for the anti-inflammatory and cytoprotective actions of ethyl pyruvate and related compounds.Biochem Pharmacol.2010;80:151- 159.
[4] Hollenbach M,Hintersdorf A,Huse K,et al.Ethyl pyruvate and ethyl lactate down-regulate the production of pro-inflammatory cytokines and modulate expression of immune receptors.Biochem Pharmacol.2008;76:631-644.
[5] Yang R,Shaufl AL,Killeen ME,et al.Ethyl pyruvate ameliorates liver injury secondary to severe acute pancreatitis.J Surg Res. 2009;153(2):302-309.
[6] Tao JY,Zheng GH,Zhao L,et al.Anti-inflammatory effects of ethyl acetate fraction from Melilotus suaveolens Ledeb on LPS-stimulated RAW 264.7 cells.J Ethnopharmacol.2009; 123:97-105.
[7] Fink MP.Ethyl pyruvate: a novel anti-inflammatory agent.J Intern Med.2007;261:349-362.
[8] 杨涛,颜光涛,司艺玲,等.丙酮酸乙酯对缺氧_复氧损伤小鼠腹腔巨噬细胞一氧化氮生成及白细胞介素-1和6表达的影响[J].感染、炎症、修复,2009,10(1):30-33.
[9] 冠秋野,管向东.丙酮酸乙酯对脓毒性休克犬氧代谢及组织灌注指标的影响[J].中国危重病急救医学,2008,20(1):34-36.
[10] 杨恒,张启瑜,朱椰凡,等.丙酮酸乙酯在大鼠重症急性胰腺炎肝损伤的作用机制[J].中华肝胆外科杂志,2011,17(6):509-511.
[11] 王瑞晨,胡森.丙酮酸乙酯减轻酵母多糖诱导的小鼠全身炎症损害[J].中国危重病急救医学,2009,21(1):43.
[12] Chaudhury A,Das S.Recent advancement of chitosan-based nanoparticles for oral controlled delivery of insulin and other therapeutic agents.AAPS Pharm Sci Tech.2011;12:10-20.
[13] Mahjub R,Dorkoosh FA,Amini M,et al.Preparation, statistical optimization, and in vitro characterization of insulin nanoparticles composed of quaternized aromatic derivatives of chitosan.AAPS Pharm Sci Tech.2011;12:1407-1419.
[14] 蒋青桃,冯旰珠,夏梅,等.结核杆菌ESAT_6抗原Th1优势表位与FL重组纳米疫苗的制备及其特性研究[J].南京医科大学学报:自然科学版,2011,31(5):620-623.
[15] 陈孟婕,姚晋荣,邵正中,等.基于生物大分子的纳米药物载体[J].化学进展,2011,23(1):202-212.
[16] Ji J,Hao S,Wu D,et al.Preparation, characterization and in vitro release of chitosan nanoparticles loaded with gentamicin and salicylic acid. Carbohydrate Polymers.2011;85:803-808.
[17] 刘霞,李伟,范小娜,等.紫外分光光度法测定普卢利沙星胶囊的含量[J].光谱实验室,2011,28(1):244-246.
[18] 张华,张卿,贾运涛,等.紫外分光光度法测定姜黄素白蛋白纳米混悬剂的包封率[J].中国药房,2012,23(43):4068-4070.
[19] 黄源锐,刘静,贾运涛,等.紫外分光光度法测定不同释放介质中5-氨基水杨酸[J].光谱实验室,2013,30(1):194-198.
[20] 李治洪.注射用更昔洛韦的HPLC测定[J].中国医药工业杂志, 2003, 34(1):30-32.
[21] 刘世军,崔晞,孙克朋,等.HPLC-荧光法研究二巯基丁二酸在小鼠体内的组织分布特性[J].中国药房,2011,22(5):413-415.
[22] 陈寅,王源,朱墨,等.HPLC法测定荧光素钠注射液含量和有关物质[J].药物分析杂志,2008,28(6):961-963.
[23] 吴公平,殷帅,李昌亮,等.气相色谱法测定2_6-二叔丁基对甲酚的含量[J].药物分析杂志,2012,32(5):865-867.
[24] 陈赞民,陈颖江.气相色谱法测定氨茶碱及其制剂中乙二胺的含量[J].药物分析杂志,2012,32(9):1673-1675.
[25] 丛英,苏佦僮,邹文銓,等.气相色谱法同时测定吐温80中7种脂肪酸亲油基的含量[J].药物分析杂志,2011,31(4):651-653.
[26] 陶国忠,郭耘,卢冠忠,等.乳酸乙酯对映体和丙酮酸乙酯混合物的气相色谱分析[J].分析化学,2007,35(3):447-450.
[27] 彭洁,王钊,李晓晔,等.GC法测定丙酮酸乙酯注射液中EP的含量[J].现代生物医学进展,2012,12(15):2822-2825.
[28] 李延志,孙昱,张春枝,等.丙酮酸乙酯的微囊化与微囊中含量检测[J].安徽农业科学,2010,38(36):20518-20520.
[29] Gupta D,Du Y,Piluek J,et al.Ethyl pyruvate ameliorates endotoxin-induced corneal inflammation.Invest Ophthalmol Vis Sci.2012;53(10):6589-6599.
[30] Kalariya NM,Reddy AB,Ansari NH,et al.Preventive effects of ethyl pyruvate on endotoxin-induced uveitis in rats.Invest Ophthalmol Vis Sci.2011;52:5144-5152.
[31] Harvey SA,Guerriero E,Charukamnoetkanok N,et al.Responses of cultured human keratocytes and myofibroblasts to ethyl pyruvate: a microarray analysis of gene expression.Invest Ophthalmol Vis Sci.2010;51: 2917-2927.
[32] Varma SD,Hegde KR,Kovtun S.Oxidative damage to lens in culture: reversibility by pyruvate and ethyl pyruvate. Ophthalmologica.2006;220(1):52-57.
[33] Reade MC,Fink MP.Bench-to-bedside review: Amelioration of acute renal impairment using ethyl pyruvate.Crit Care.2005;9: 556-560. |