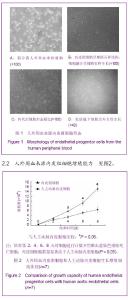
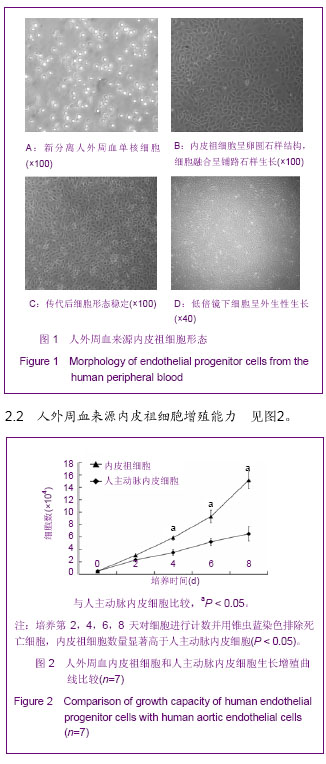
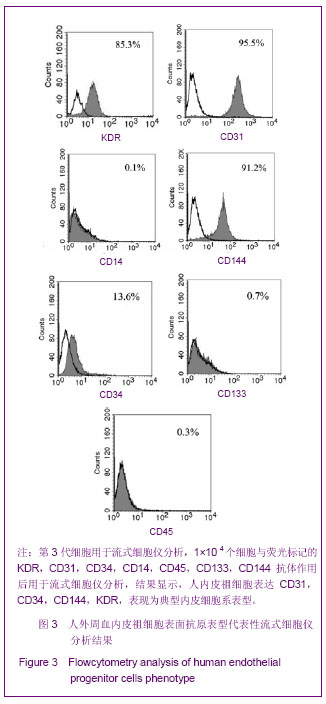
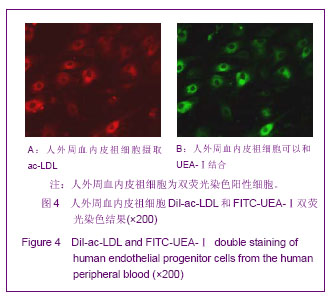
| [1] Resch T, Pircher A, Kähler CM, et al. Endothelial progenitor cells: current issues on characterization and challenging clinical applications.Stem Cell Rev. 2012;8(3):926-939.
[2] 陆晶晶,杨振东,王桂敏.内皮祖细胞在血管相关疾病治疗中的作用[J].中国组织工程研究与临床康复,2010, 14(27):5103-5106.
[3] Li DW, Liu ZQ, Wei J, et al. Contribution of endothelial progenitor cells to neovascularization. Int J Mol Med. 2012; 30(5):1000-1006.
[4] Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275(5302):964-967.
[5] Devanesan AJ,Laughlan KA,Girn HR,et al.Endothelial progenitor cells as a therapeutic option in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2009;38(4): 478-481.
[6] Xue S, Zhang HT , Zhang P, et al. Functional endothelial progenitor cells derived from adipose tissue show beneficial effect on cell therapy of traumatic brain injury. Neurosci Lett. 2010;473(3):186-191.
[7] Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, et al. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res. 2010;80(3):317-323.
[8] 施森,何延政, 刘勇.内皮祖细胞在血管组织工程中的应用[J]. 中国组织工程研究与临床康复, 2007,11(7):1325-1328.
[9] George AL, Bangalore-Prakash P, Rajoria S, et al. Endothelial progenitor cell biology in disease and tissue regeneration. J Hematol Oncol. 2011;24;4-24.
[10] Timmermans F, Plum J, Yoder MC, et al. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009; 13(1): 87-102.
[11] 刘隽炜,董念国. 内皮祖细胞研究进展[J]. 心血管病学进展, 2011, 32(4):528-531.
[12] Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5): 1801-1809.
[13] Yoder MC. Defining human endothelial progenitor cells. J Thromb Haemost. 2009; 7(Suppl. 1): 49-52.
[14] Richardson MR, Yoder MC. Endothelial progenitor cells: Quo Vadis?J Mol Cell Cardiol. 2011; 50(2): 266-272.
[15] Mead LE, Prater D, Yoder MC, et al. Isolation and characterization of endothelial progenitor cells from human blood.CurrProtoc Stem Cell Biol. 2008;Chapter 2:Unit 2C.1.
[16] Tura O, Skinner EM, Barclay GR, et al. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. Stem Cells. 2013;31(2): 338-348.
[17] Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221-228.
[18] Bajpai VK, Andreadis ST. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng Part B Rev. 2012;18(5):405-425.
[19] Smadja DM, Cornet A, Emmerich J, et al. Endothelial progenitor cells: characterization, in vitro expansion, and prospects for autologous cell therapy. Cell Biol Toxicol. 2007; 3(4):223-239.
[20] 刘冉,兰玲,于静.内皮祖细胞研究进展[J].中华实用诊断与治疗杂志, 2011,25(7):628-630.
[21] Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004; 95(4): 343-353.
[22] 方立建,宋鄂,栾瑛,等.人脐带血内皮祖细胞体外培养和鉴定[J].中国组织工程研究与临床康复,2010,14(45):8450-8454.
[23] 徐燕,孟恒星,于珍,等.不同来源内皮祖细胞培养及其生物学特性比较[J].中国组织工程研究与临床康复,2010,14(23):4309-4316.
[24] 周秀娟,闫醒军,何延政,等.人外周血、脐血、脂肪组织来源的内皮祖细胞部分生物学特性比较[J].中国动脉硬化杂志,2011, 19(8):675-679.
[25] Zhang M, Huang B. The multi-differentiation potential of peripheral blood mononuclear cells. Stem Cell Res Ther. 2012;3(6):48.
[26] Favre J, Terborg N, Horrevoets AJ. The diverse identity of angiogenic monocytes. Eur J Clin Invest. 2013;43(1): 100-107.
[27] Kässmeyer S, Plendl J, Custodis P, et al.New insights in vascular development: vasculogenesis and endothelial progenitor cells.Anat Histol Embryol. 2009;38(1):1-11.
[28] Mukai N, Akahori T, Komaki M, et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2008;314(3):430-440.
[29] Medina RJ, O'Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18.
[30] Napoli C, Hayashi T, Cacciatore F, et al. Endothelial progenitor cells as therapeutic agents in the microcirculation: an update.Atherosclerosis.2011;215(1):9-22.
[31] Pearson JD. Endothelial progenitor cells--an evolving story. Micro Vasc Res. 2010;79(3):162-168.
[32] Bouchentouf M, Forner K, Cuerquis J, et al. A novel and simplified method of culture of human blood-derived early endothelial progenitor cells for the treatment of ischemic vascular disease. Cell Transplant. 2011;20(9):1431-1443.
[33] Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors.Arterioscler Thromb Vasc Biol. 2007;27(7):1572-1579.
[34] 李华,高建华,颜玲,等.成人外周血单个核细胞体外培养过程中分化为早及晚期内皮祖细胞生物学表征[J].中国组织工程研究与临床康复,2007,11(24):4682-4685.
[35] Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood, 2005,106(5):1525-1531.
[36] Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol. 2007;292(1):H1-18.
[37] Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl). 2013;91(3):285-295.
[38] Brown MA, Wallace CS, Angelos M, et al. Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A. 2009;15(11):3575-3587.
[39] Prasain N, Meador JL, Yoder MC. Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J Vis Exp. 2012; 62: pii: 3872.
[40] Strunk D. Endothelial progenitor cells: quod erat demonstrandum? Curr Pharm Des. 2011;17(30):3245-3251. |