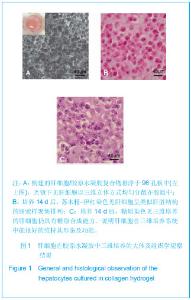
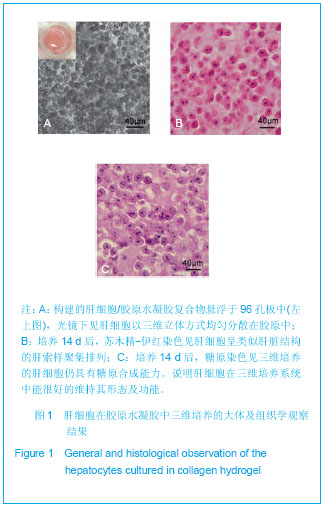
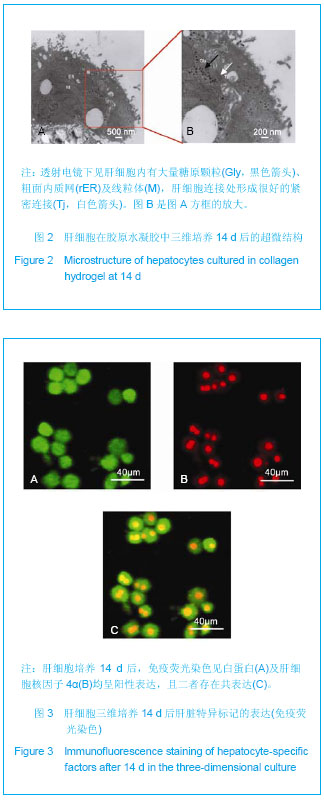
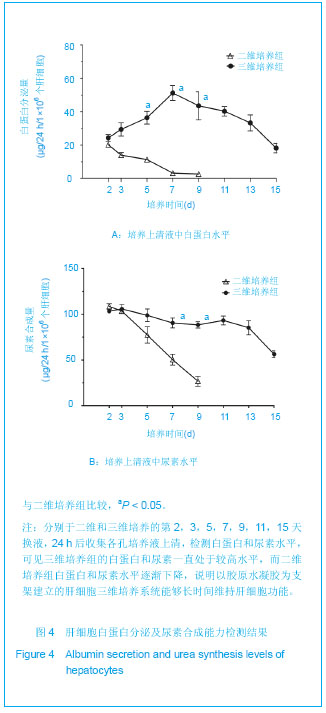
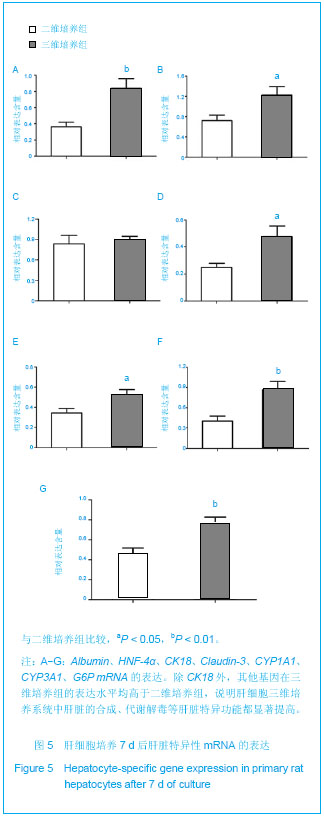
Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (29): 5323-5330.doi: 10.3969/j.issn.2095-4344.2013.29.010
Previous Articles Next Articles
In vitro establishment of a three-dimensional hepatocyte culture system using collagen hydrogel as scaffolds
Wang Min1, Lan Ya2, Hu Hao1, Shi Yong-quan1, Han Ying1, Zhou Xin-min1
- 1State Key Laboratory of Cancer Biology, Xijing Hospital of Digestive Diseases, the Fourth Military Medical University of PLA, Xi’an 710032, Shaanxi Province, China; 2Department of Gastroenterology, Yan’an University Affiliated Hospital, Yan’an 716000, Shaanxi Province, China
-
Received:2013-03-19Revised:2013-04-14Online:2013-07-22Published:2013-07-22 -
Contact:Zhou Xin-min, M.D., Professor, Chief physician, State Key Laboratory of Cancer Biology, Xijing Hospital of Digestive Diseases, the Fourth Military Medical University of PLA, Xi’an 710032, Shaanxi Province, China zhouxm128@fmmu.edu.cn -
About author:Wang Min★, Studying for master’s degree, State Key Laboratory of Cancer Biology, Xijing Hospital of Digestive Diseases, the Fourth Military Medical University of PLA, Xi’an 710032, Shaanxi Province, China wangminfmmu@126.com -
Supported by:the Science and Technology Innovation Engineering Plan of Shaanxi Province, No. 2012KTCL03-04*
CLC Number:
Cite this article
Wang Min, Lan Ya, Hu Hao, Shi Yong-quan, Han Ying, Zhou Xin-min. In vitro establishment of a three-dimensional hepatocyte culture system using collagen hydrogel as scaffolds[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(29): 5323-5330.
share this article
| [1] Xia L, Sakban RB, Qu Y, et al. Tethered spheroids as an in vitro hepatocyte model for drug safety screening. Biomaterials. 2012;33(7):2165-2176.http://www.ncbi.nlm.nih.gov/pubmed/?term=Tethered+spheroids+as+an+in+vitro+hepatocyte+model+for+drug+safety+screening.[2]Bierwolf J, Lutgehetmann M, Feng K, et al. Primary rat hepatocyte culture on 3D nanofibrous polymer scaffolds for toxicology and pharmaceutical research. Biotechnol Bioeng. 2011;108(1):141-150.http://www.ncbi.nlm.nih.gov/pubmed/?term=Primary+rat+hepatocyte+culture+on+3D+nanofibrous+polymer+scaffolds+for+toxicology+and+pharmaceutical+research.[3] Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci U S A. 2007;104(14):5722-5726.http://www.ncbi.nlm.nih.gov/pubmed/17389399[4] Ohashi K, Yokoyama T, Yamato M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13(7):880-885.http://www.ncbi.nlm.nih.gov/pubmed/?term=Engineering+functional+two-+and+three-dimensional+liver+systems+in+vivo+using+hepatic+tissue+sheets.[5] Kim K, Ohashi K, Utoh R, et al. Preserved liver-specific functions of hepatocytes in 3D co-culture with endothelial cell sheets. Biomaterials. 2012;33(5):1406-1413.http://www.ncbi.nlm.nih.gov/pubmed/?term=Preserved+liver-specific+functions+of+hepatocytes+in+3D+co-culture+with+endothelial+cell+sheets.[6] Rhim JA, Sandgren EP, Degen JL, et al. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263(5150):1149-1152.http://www.ncbi.nlm.nih.gov/pubmed/8108734[7] Ohashi K, Park F, Kay MA. Hepatocyte transplantation: clinical and experimental application. J Mol Med (Berl). 2001;79(11):617-630.http://www.ncbi.nlm.nih.gov/pubmed/?term=J+Mol+Med+(Berl).+2001%3B+11(79)%3A617-630[8] Goto Y, Ohashi K, Utoh R, et al. Hepatocyte transplantation through the hepatic vein: a new route of cell transplantation to the liver. Cell Transplant. 2011;20(8):1259-1270.http://www.ncbi.nlm.nih.gov/pubmed/?term=Hepatocyte+transplantation+through+the+hepatic+vein%3A+a+new+route+of+cell+transplantation+to+the+liver.[9] Carpentier B, Gautier A, Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58(12):1690-1702.http://www.ncbi.nlm.nih.gov/pubmed/19923348[10] lin JM, Kneip B, Vaulont S, et al. Dependence of hepatocyte-specific gene expression on cell-cell interactions in primary culture. EMBO J. 1985;4(10):2487-2491.http://www.ncbi.nlm.nih.gov/pubmed/?term=Dependence+of+hepatocyte-specific+gene+expression+on+cell-cell+interactions+in+primary+culture.[11] Ben-Ze'ev A, Robinson GS, Bucher NL, et al. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988;85(7):2161-2165.http://www.ncbi.nlm.nih.gov/pubmed/?term=Cell-cell+and+cell-matrix+interactions+differentially+regulate+the+expression+of+hepatic+and+cytoskeletal+genes+in+primary+cultures+of+rat+hepatocytes.[12] Oda H, Yoshida Y, Kawamura A, et al. Cell shape, cell-cell contact, cell-extracellular matrix contact and cell polarity are all required for the maximum induction of CYP2B1 and CYP2B2 gene expression by phenobarbital in adult rat cultured hepatocytes. Biochem Pharmacol. 2008;75(5):1209-1217.http://www.ncbi.nlm.nih.gov/pubmed/?term=Biochem+Pharmacol.+2008%3B+5(75)%3A1209-1217[13] Mooney D, Hansen L, Vacanti J, et al. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151(3):497-505.http://www.ncbi.nlm.nih.gov/pubmed/1295898[14] Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7(3):237-245.http://www.ncbi.nlm.nih.gov/pubmed/?term=Long-term+in+vitro+function+of+adult+hepatocytes+in+a+collagen+sandwich+configuration.[15] Dunn JC, Yarmush ML, Koebe HG, et al. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3(2):174-177.http://www.ncbi.nlm.nih.gov/pubmed/?term=FASEB+J.+1989%3B+2(3)%3A174-177[16] Wu FJ, Friend JR, Hsiao CC, et al. Efficient assembly of rat hepatocyte spheroids for tissue engineering applications. Biotechnol Bioeng. 1996;50(4):404-415.http://www.ncbi.nlm.nih.gov/pubmed/?term=Efficient+assembly+of+rat+hepatocyte+spheroids+for+tissue+engineering+applications.[17] Pulavendran S, Rose C, Mandal AB. Hepatocyte growth factor incorporated chitosan nanoparticles augment the differentiation of stem cell into hepatocytes for the recovery of liver cirrhosis in mice. J Nanobiotechnology. 2011;9:15.http://www.ncbi.nlm.nih.gov/pubmed/?term=Hepatocyte+growth+factor+incorporated+chitosan+nanoparticles+augment+the+differentiation+of+stem+cell+into+hepatocytes+for+the+recovery+of+liver+cirrhosis+in+mice.[18] Ananthanarayanan A, Narmada BC, Mo X, et al. Purpose-driven biomaterials research in liver-tissue engineering. Trends Biotechnol. 2011;29(3):110-118.http://www.ncbi.nlm.nih.gov/pubmed/?term=Purpose-driven+biomaterials+research+in+liver-tissue+engineering.[19] Feng ZQ, Chu X, Huang NP, et al. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials. 2009;30(14):2753-2763.http://www.ncbi.nlm.nih.gov/pubmed/?term=Biomaterials.+2009%3B+14(30)%3A2753-2763[20] Cushing MC, Anseth KS. Materials science. Hydrogel cell cultures. Science. 2007;316(5828):1133-1134.http://www.ncbi.nlm.nih.gov/pubmed/?term=Hydrogel+cell+cultures.+Science.+2007%3B+5828(316)%3A1133-1134[21] Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124-1128.http://www.ncbi.nlm.nih.gov/pubmed/?term=Designing+cell-compatible+hydrogels+for+biomedical+applications.[22] Kim M, Lee JY, Jones CN, et al. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31(13):3596-3603.http://www.ncbi.nlm.nih.gov/pubmed/?term=Heparin-based+hydrogel+as+a+matrix+for+encapsulation+and+cultivation+of+primary+hepatocytes.[23] Fu Y, Xu K, Zheng X, et al. 3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 2012;33(1):48-58.http://www.ncbi.nlm.nih.gov/pubmed/?term=Biomaterials.+2012%3B+1(33)%3A48-58[24] Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3(1):a004978.http://www.ncbi.nlm.nih.gov/pubmed/21421911[25] Friess W. Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113-136.http://www.ncbi.nlm.nih.gov/pubmed/?term=European+Journal+of+Pharmaceutics+and+Biopharmaceutics.+1998%3B+2(45)%3A113-136[26] Nagamoto Y, Tashiro K, Takayama K, et al. The promotion of hepatic maturation of human pluripotent stem cells in 3D co-culture using type I collagen and Swiss 3T3 cell sheets. Biomaterials. 2012;33(18):4526-4534.http://www.ncbi.nlm.nih.gov/pubmed/?term=The+promotion+of+hepatic+maturation+of+human+pluripotent+stem+cells+in+3D+co-culture+using+type+I+collagen+and+Swiss+3T3+cell+sheets.[27] Yuan T, Zhang L, Feng L, et al. Chondrogenic differentiation and immunological properties of mesenchymal stem cells in collagen type I hydrogel. Biotechnol Prog. 2010;26(6):1749-1758.http://www.ncbi.nlm.nih.gov/pubmed/?term=Biotechnol+Prog.+2010%3B+6(26)%3A1749-1758[28] Zheng L, Sun J, Chen X, et al. In vivo cartilage engineering with collagen hydrogel and allogenous chondrocytes after diffusion chamber implantation in immunocompetent host. Tissue Eng Part A. 2009;15(8):2145-2153.http://www.ncbi.nlm.nih.gov/pubmed/?term=In+vivo+cartilage+engineering+with+collagen+hydrogel+and+allogenous+chondrocytes+after+diffusion+chamber+implantation+in+immunocompetent+host.[29] Oh SA, Lee HY, Lee JH, et al. Collagen three-dimensional hydrogel matrix carrying basic fibroblast growth factor for the cultivation of mesenchymal stem cells and osteogenic differentiation. Tissue Eng Part A. 2012;18(9-10):1087-1100.http://www.ncbi.nlm.nih.gov/pubmed/?term=Three-Dimensional+Hydrogel+Matrix+Carrying+Basic+Fibroblast+Growth+Factor+for+the+Cultivation+of+Mesenchymal+Stem+Cells+and+Osteogenic+Differentiation.[30] Zhao Y, Xu Y, Zhang B, et al. In vivo generation of thick, vascularized hepatic tissue from collagen hydrogel-based hepatic units. Tissue Eng Part C Methods. 2010;16(4):653-659.http://www.ncbi.nlm.nih.gov/pubmed/?term=In+vivo+generation+of+thick%2C+vascularized+hepatic+tissue+from+collagen+hydrogel-based+hepatic+units.[31] Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83.http://www.ncbi.nlm.nih.gov/pubmed/?term=Seglen+PO.+Preparation+of+isolated+rat+liver+cells.+Methods+Cell+Biol+1976%3B13%3A[32] Zhao YS, Xu YX, Zhang BF, et al. Experimental study of implantable engineered liver tissue using type I collagen gel as scaffold. Zhonghua Yi Xue Za Zhi. 2007;87(29):2065-2068.http://www.ncbi.nlm.nih.gov/pubmed/?term=Experimental+study+of+implantable+engineered+liver+tissue+using+type+I+collagen+gel+as+scaffold.[33] Wang S, Nagrath D, Chen PC, et al. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng Part A. 2008;14(2):227-236.http://www.ncbi.nlm.nih.gov/pubmed/?term=Three-dimensional+primary+hepatocyte+culture+in+synthetic+self-assembling+peptide+hydrogel.[34] Saha K, Pollock JF, Schaffer DV, et al. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11(4):381-387.http://www.ncbi.nlm.nih.gov/pubmed/?term=Designing+synthetic+materials+to+control+stem+cell+phenotype[35] Levenberg S, Huang NF, Lavik E, et al. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003;100(22):12741-12746.http://www.ncbi.nlm.nih.gov/pubmed/14561891[36] Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655-663.http://www.ncbi.nlm.nih.gov/pubmed/19472329[37] Shea LD, Smiley E, Bonadio J, et al. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17(6):551-554.http://www.ncbi.nlm.nih.gov/pubmed/10385318[38] Semino CE, Merok JR, Crane GG, et al. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003;71(4-5):262-270.http://www.ncbi.nlm.nih.gov/pubmed/?term=Differentiation.+2003%3B+4-5(71)%3A262-270[39] Silva GA, Czeisler C, Niece KL, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352-1355.http://www.ncbi.nlm.nih.gov/pubmed/?term=Selective+differentiation+of+neural+progenitor+cells+by+high-epitope+density+nanofibers.[40] Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):149-165.http://www.ncbi.nlm.nih.gov/pubmed/18498217[41] Egawa EY, Kato K, Hiraoka M, et al. Enhanced proliferation of neural stem cells in a collagen hydrogel incorporating engineered epidermal growth factor. Biomaterials. 2011;32(21):4737-4743.http://www.ncbi.nlm.nih.gov/pubmed/?term=Enhanced+proliferation+of+neural+stem+cells+in+a+collagen+hydrogel+incorporating+engineered+epidermal+growth+factor[42] Hammond JS, Gilbert TW, Howard D, et al. Scaffolds containing growth factors and extracellular matrix induce hepatocyte proliferation and cell migration in normal and regenerating rat liver. J Hepatol. 2011;54(2):279-287.http://www.ncbi.nlm.nih.gov/pubmed/?term=J+Hepatol.+2011%3B+2(54)%3A279-287[43] Sellaro TL, Ranade A, Faulk DM, et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;16(3):1075-1082.http://www.ncbi.nlm.nih.gov/pubmed/?term=Maintenance+of+human+hepatocyte+function+in+vitro+by+liver-derived+extracellular+matrix+gels.[44] Berthiaume F, Moghe PV, Toner M, et al. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10(13):1471-184.http://www.ncbi.nlm.nih.gov/pubmed/?term=Effect+of+extracellular+matrix+topology+on+cell+structure%2C+function%2C+and+physiological+responsiveness+hepatocytes+cultured+in+a+sandwich+configuration.[45] Page JL, Johnson MC, Olsavsky KM, et al. Gene expression profiling of extracellular matrix as an effector of human hepatocyte phenotype in primary cell culture. Toxicol Sci. 2007;97(2):384-397.http://www.ncbi.nlm.nih.gov/pubmed/?term=Gene+expression+profiling+of+extracellular+matrix+as+an+effector+of+human+hepatocyte+phenotype+in+primary+cell+culture.[46] Zavan B, Brun P, Vindigni V, et al. Extracellular matrix-enriched polymeric scaffolds as a substrate for hepatocyte cultures: in vitro and in vivo studies. Biomaterials. 2005;26(34):7038-7045.http://www.ncbi.nlm.nih.gov/pubmed/?term=Extracellular+matrix-enriched+polymeric+scaffolds+as+a+substrate+for+hepatocyte+cultures%3A+in+vitro+and+in+vivo+studies.+Biomaterials.[47] Shen C, Meng Q. Prediction of cytochrome 450 mediated drug-drug interactions by three-dimensional cultured hepatocytes. Mini Rev Med Chem. 2012;12(10):1028-1036.http://www.ncbi.nlm.nih.gov/pubmed/?term=Prediction+of+cytochrome+450+mediated+drug-drug+interactions+by+three-dimensional+cultured+hepatocytes.[48] Li J, Ning G, Duncan SA. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev. 2000;14(4):464-474.http://www.ncbi.nlm.nih.gov/pubmed/10691738[49] Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part II: the C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol Rev. 2004;56(2):291-330.http://www.ncbi.nlm.nih.gov/pubmed/?term=Pharmacol+Rev.+2004%3B+2(56)%3A291-330[50] Dean S, Tang JI, Seckl JR, et al. Developmental and tissue-specific regulation of hepatocyte nuclear factor 4-alpha (HNF4-alpha) isoforms in rodents. Gene Expr. 2010;14(6):337-344.http://www.ncbi.nlm.nih.gov/pubmed/?term=Developmental+and+tissue-specific+regulation+of+hepatocyte+nuclear+factor+4-alpha+(HNF4-alpha)+isoforms+in+rodents.[51] Takaki Y, Hirai S, Manabe N, et al. Dynamic changes in protein components of the tight junction during liver regeneration. Cell Tissue Res. 2001;305(3):399-409.http://www.ncbi.nlm.nih.gov/pubmed/?term=Dynamic+changes+in+protein+components+of+the+tight+junction+during+liver+regeneration.[52] Sawada N, Murata M, Kikuchi K, et al. Tight junctions and human diseases. Med Electron Microsc. 2003;36(3):147-156.http://www.ncbi.nlm.nih.gov/pubmed/?term=Med+Electron+Microsc.+2003%3B+3(36)%3A147-156[53] Bhatia SN, Balis UJ, Yarmush ML, et al. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883-1900.http://www.ncbi.nlm.nih.gov/pubmed/10544172[54] Nishikawa M, Kojima N, Komori K, et al. Enhanced maintenance and functions of rat hepatocytes induced by combination of on-site oxygenation and coculture with fibroblasts. J Biotechnol. 2008;133(2):253-260.http://www.ncbi.nlm.nih.gov/pubmed/?term=Enhanced+maintenance+and+functions+of+rat+hepatocytes+induced+by+combination+of+on-site+oxygenation+and+coculture+with+fibroblasts.[55] Zinchenko YS, Culberson CR, Coger RN. Contribution of non-parenchymal cells to the performance of micropatterned hepatocytes. Tissue Eng. 2006;12(8):2241-2251.http://www.ncbi.nlm.nih.gov/pubmed/?term=Contribution+of+Non-parenchymal+Cells+to+the+Performance+of+Micropatterned+Hepatocytes.+Tissue+Eng+Part+A.+2006%3B |
| [1] | Bai Wen-yuan, Gu Hong-sheng, Liao Zhen-hua, Liu Wei-qiang. Clinical application of artificial lumbar disc replacement: Present and future [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(35): 6321-6326. |
| [2] | Li Rui-qi, Zhang Guo-ping, Ren Li-zhong, Li Ya-li, Lü Ya-jun. Evaluation of bone marrow mesenchymal stem cells for the treatment of osteonecrosis of femoral head [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(35): 6327-6332. |
| [3] | Chen Yue-ping, Gao Hui, Chen Liang, Dong Pan-feng, Yin Qing-shui. Alcohol affects the femoral head intramedullary adipocytes [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(35): 6221-6227. |
| [4] | Xie Ming-quan, Liu Hui-juan, Li Ping, Li Yu-min. Preparation and properties for magnetic/pH double sensitive hydrogel beads carrying carbamazepine [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(29): 5337-5344. |
| [5] | Bao Yu-cheng, Zhang Wen-long, Wang Yong, Zhang Jie, Wang Yong-mei. Dual-drug sustained-release carrier: Preparation and performance [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(29): 5345-5350. |
| [6] | Ye Peng, Tian Ren-yuan, Huang Wen-liang, Ma Li-kun, Deng Jiang. Silk fibroin/chitosan/nano hydroxyapatite complicated scaffolds for bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(29): 5269-5274. |
| [7] | Ma Lu-ping, Zhong Liang-jun, Zhang Yuan-ming, Zhang Yuan, Zhang Peng-tao. Periodontal ligament cells from miniature swine grow on a hydroxyapatite scaffold [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(29): 5290-5295. |
| [8] | Lu Feng, Zhang Hong-tu, Ma Shu-you. Drug release characteristics of gatifloxacin-poly sebacic anhydride local controlled release system [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(29): 5351-5356. |
| [9] | Xian Yuan-fang, Wang Wen-ting, Yu Wei, Tu Li-hui, Wang Sheng-hai, Zou Cheng, Min Xiao-feng. Preparation of gelatin-magnetic micro-capsules by condensation method [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(29): 5357-5363. |
| [10] | Lu Shou-ming, Lu Shou-liang, Sun Tian-wei, Zhang Hang, Wang Qi-ming. Estrogen effects on serum interleukin-8 and interleukin-10 expression in ovariectomized rats with osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(24): 4394-4400. |
| [11] | Adila•Azhati, Zhao Long, Wang Qian, Ma Yi-tong. Cardiac function and electrophysiological characteristics in myocardial infarction rats after tissue engineered cardiac patch transplantation [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(24): 4401-4408. |
| [12] | Shi Yu-lu, Li Xiao-yuan, Cao Mei-na, Yu Shu-yuan, Wang Ping. Simultaneous isolation of myocardial cells and cardiac fibroblasts from neonatal rats [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(24): 4414-4420. |
| [13] | Li Wei-hua, Gao Yu-wei, Li De-shui, Xing Yu-xi. Botulinum toxin type A induces apoptosis of human hypertrophic scar fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(24): 4429-4435. |
| [14] | Chen Dan-dan, Zhou Da-xin, Guan Li-hua, Chen Fa-dong, Dong Li-li, Qian Ju-ying, Ge Jun-bo. Application of continuous thermodilution method in beagle models with pulmonary arterial hypertension [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(24): 4509-4514. |
| [15] | Zhao Zhong-fu, Fan Rui-tai, Yang Yong, Sun Jian-rui, Hu Xiang, Eileen Shen, Yang Bo. In vitro radiosensitivity of brain cancer stem cells in brain glioma [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(19): 3455-3460. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||