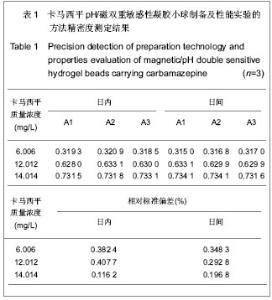
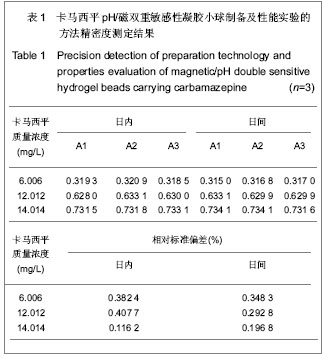
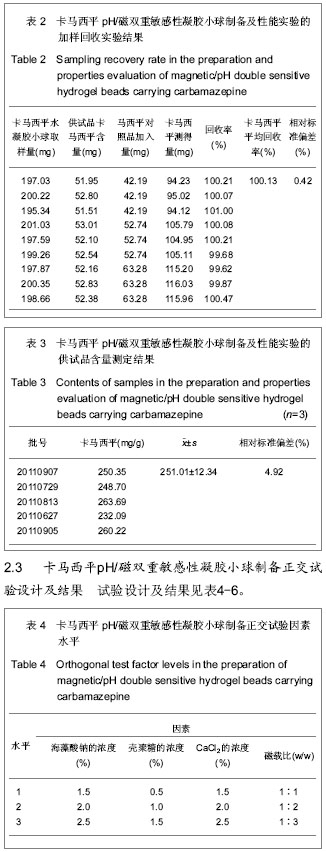
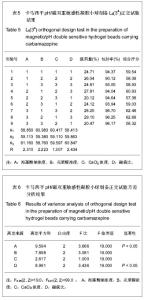
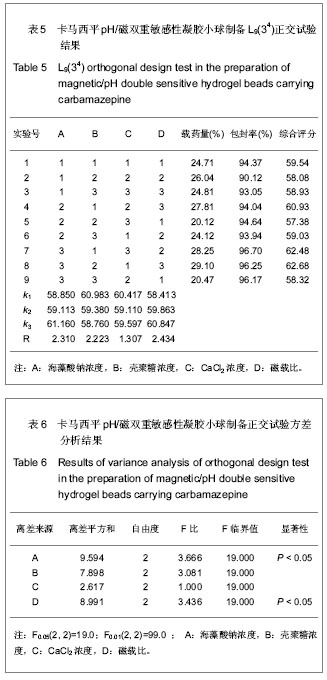
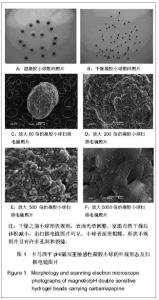
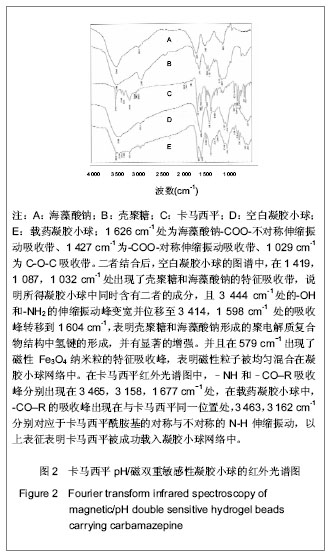
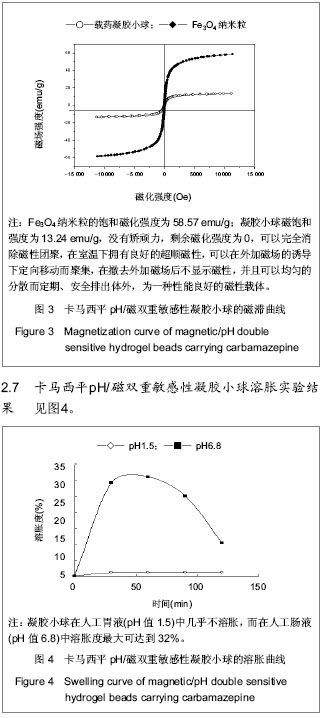
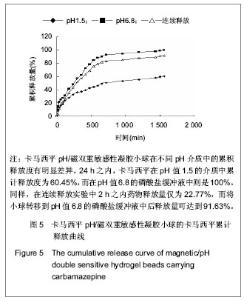
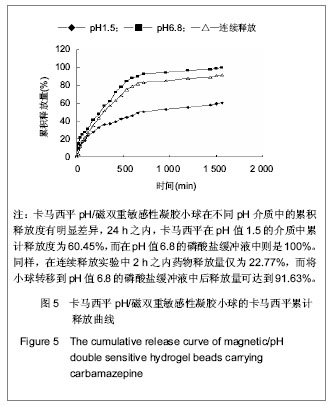
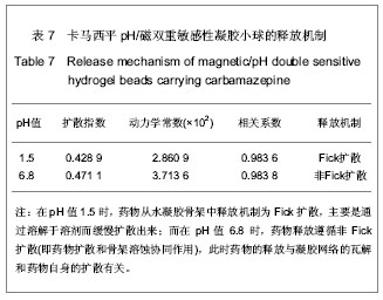
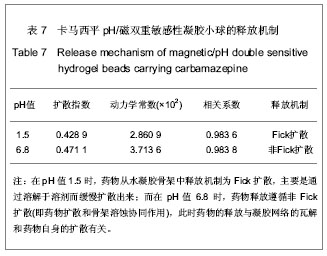
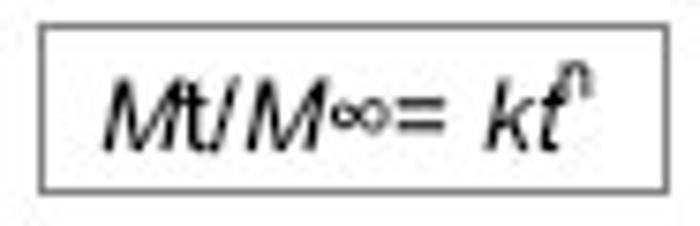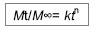| [1] 焦正,赵志刚,施孝金,等.比较HPLC和FPIA法测定人血清中卡马西平的血药浓度[J].中国临床药理学杂志,2004,20(4):291-294.[2] 陈泽莲,徐瑛,吴逢波,等.卡马西平不良反应的临床特征及相关因素分析[J].中国药房,2007,18(11):864-866.[3] 席枝侠,仵文英,马小亚,等.卡马西平固体脂质纳米粒的包封率测定[J].中国药房,2008,19(13):1009-1011.[4] 中国药典. 二部[S].2010:126-127.[5] Liu HJ,Li P,Wei Q.Magnetic N-succinyl chitosan/alginate beads for carbamazepine delivery. Drug Dev Ind Pharm. 2010;36(11): 1286-1294.[6] Sankalia MG,Mashru RC,Sankalia JM,et al.Reversed chitosan–alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization.Eur J Pharm Biopharm. 2007;65(2): 215-232.[7] Chen SC,Wu YC,Mi FL,et al.A novel pH-sensitive hydrogel composed of N, O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery.J Control Release.2004;96 (2): 285-300.[8] Li S,Wang XT,Zhang XB,et al.Studies on alginate–chitosan microcapsules and renal arterial embolization in rabbits.J Control Release.2002;84(3): 87-98.[9] Yang WC,Xie R,Pang XQ,et al.Preparation and characterization of dual stimuli-responsive microcapsules with a superparamagnetic porous membrane and thermo-responsive gates.J Memb Sci.2008;321(2): 324-330.[10] Bouropoulos PG.Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads.Int J Pharm.2006;323(1-2): 34-42.[11] Xu Y,Zhan CY,Fan LH,et al.Physicochemical properties and rheology of alginate gel beads formed with various divalent cations. Int J Pharm. 2007;336: 329-337.[12] 邢楠,田丰木,刘圣军,等.海藻酸钙-壳聚糖微胶囊组成对BSA通透性能影响的研究[J].化学学报,2007,65(24):2952-2958.[13] Wong TW,Chan LW,Kho SB,et al.Design of controlled-release solid dosage forms of alginate and chitosan using microwave.J Control Release. 2002;84(3): 99-114.[14] Sethia S,Squillante E.Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods.Int J Pharm.2004;272(1-2): 1-10. |