Chinese Journal of Tissue Engineering Research
Previous Articles Next Articles
Mesenchymal stem cells for the treatment of spinocerebellar ataxia
Hu Jing-qiong1, Ouyang Wei-xiang2, Li Hui-yu1, Wang Jun-feng1, Lu Cong1, Zhang Lan-nan1, Xu Hai-bo3, Chen Li-li4, Huang Shi-ang1
- 1Stem Cell Center, 2Department of Obstetrics and Gynecology, 3Department of Radiology, 4Dental Center, Wuhan Union Hospital, Wuhan 430022, Hubei Province, China
-
Received:2012-10-02Revised:2012-12-20Online:2013-07-02Published:2013-07-02 -
Contact:Chen Li-li, M.D., Professor, Dental Center, Wuhan Union Hospital, Wuhan 430022, Hubei Province, China Corresponding author: Huang Shi-ang, M.D., Professor, Stem Cell Center, Wuhan Union Hospital, Wuhan 430022, Hubei Province, China -
About author:Hu Jing-qiong☆, M.D., Associate professor, Stem Cell Center, Wuhan Union Hospital, Wuhan 430022, Hubei Province, China jingqionghu2006@gmail.com -
Supported by:The Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars of China*; Projects of International Cooperation and Exchanges, National Natural Science Foundation of China, No. 31110103905/C10030*; General Project of the National Natural Science Foundation of China, No. 81171386/H1808
CLC Number:
Cite this article
Hu Jing-qiong, Ouyang Wei-xiang, Li Hui-yu, Wang Jun-feng, Lu Cong, Zhang Lan-nan, Xu Hai-bo, Chen Li-li, Huang Shi-ang. Mesenchymal stem cells for the treatment of spinocerebellar ataxia[J]. Chinese Journal of Tissue Engineering Research, doi: 10.3969/j.issn.2095-4344.2013.27.012.
share this article
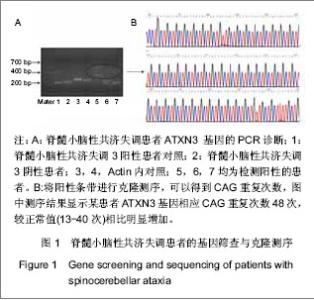
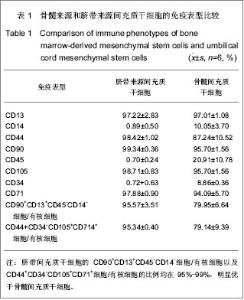
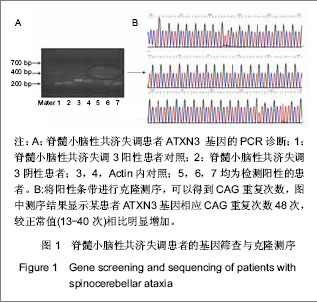
2.1 参与者数量分析 纳入经临床、头颅MRI检查确诊的27例脊髓小脑性共济失调患者,其中6例行自体骨髓间充质干细胞治疗,细胞采集以及回输均为一次性,为1个疗程;21例行异体脐带间充质干细胞输注治疗,4 次为1个疗程。患者随访时间为1年,无随访脱落,按照意向性处理分析所有病例均进入结果分析。 2.2 脊髓小脑性共济失调的基因诊断 所有患者外周血DNA均采用PCR技术扩增ATXN1,3,7基因相应CAG重复序列并以1%琼脂糖凝胶电泳。正常阴性对照产生一正常条带(约200 bp),患者PCR扩增后产物电泳可见一正常条带(约200 bp)以及一异常条带 (有时有两条),明显大于300 bp,与脊髓小脑性共济失调阳性对照条带基本吻合,提示ATXN基因相应CAG重复序列异常增加,见图1A。对阳性病例扩增出的异常条带进行克隆测序。27例病例中11例脊髓小脑性共济失调3基因出现异常扩增,ATXN3基因相应CAG重复次数为50-78次,较正常值(13-40次)相比增加,见图1B。"
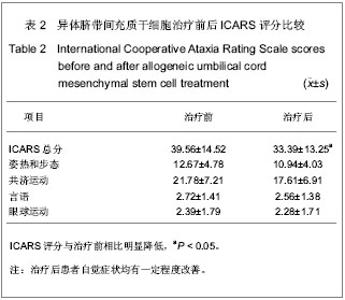
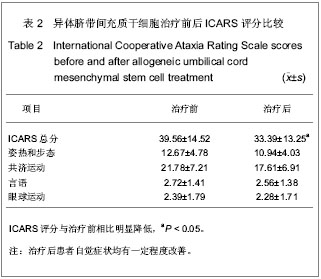
2.5 临床疗效 采用ICARS对患者治疗前后神经功能进行评定,两组患者治疗前ICARS评分大致相当,骨髓间充质干细胞治疗组治疗前ICARS(38.05±10.85)分;脐带间充质干细胞治疗组治疗前ICARS(39.56±14.52)分。6例脊髓小脑共济失调患者采用自体骨髓间充质干细胞治疗后效果均不明显,另外21例脊髓小脑共济失调患者行异体脐带间充质干细胞腰穿结合静脉输注治疗,治疗过程中以及治疗前后均未及明显不良反应,21例患者治疗前ICARS(39.56±14.52)分,治疗后1个月评分(36.28±14.76)分,差异无显著性意义。但3个月后患者自觉症状均有一定程度改善,ICARS评分(33.39±13.25)分,与治疗前相比明显降低(P < 0.05),见表2。"
| [1] Koch P, Breuer P, Peitz M, et al.Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature. 2011;480(7378):543-546. [2] Paulson H.Machado-Joseph Disease: from first descriptions to new perspectives. Handb Clin Neurol. 2012;103:437-449.[3] D'Abreu A, França MC Jr, Paulson HL, et al. New insights into the pathoanatomy of spinocerebellar ataxia type 3 (Machado-Joseph disease). Curr Opin Neurol. 2008;21(2): 111-116.[4] Yamada M, Sato T, Tsuji S, Takahashi H. CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115(1):71-86.[5] Zhang L, Tan X, Dong C, et al.In vitro differentiation of human umbilical cord mesenchymal stem cells (hUCMSCs), derived from Wharton's jelly, into choline acetyltransferase (ChAT)-positive cells. Int J Dev Neurosci. 2012;30(6): 471-477. [6] Datta I, Mishra S, Mohanty L, et al.Neuronal plasticity of human Wharton's jelly mesenchymal stromal cells to the dopaminergic cell type compared with human bone marrow mesenchymal stromal cells.Cytotherapy. 2011;13(8):918-932.[7] Zhang HT, Fan J, Cai YQ, et al. Human Wharton's jelly cells can be induced to differentiate into growth factor-secreting oligodendrocyte progenitor-like cells.Differentiation. 2010; 79(1):15-20.[8] Peng J, Wang Y, Zhang L, et al. Human umbilical cord Wharton's jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84(3):235-243.[9] Ding DC, Shyu WC, Lin S Z. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5-14.[10] Caimi PF, Reese J, Lee Z, et al. Emerging therapeutic approaches for multipotent mesenchymal stromal cells. Curr Opin Hematol. 2010;17(6):505-513. [11] Lin YC, Ko TL, Shih YH,et al.Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42(7):2045-2053.[12] Lim JY, Jeong CH, Jun JA,et al.Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia.Stem Cell Res Ther. 2011;22:2(5):38. [13] Kim ES, Ahn SY, Im GH, et al.Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats.Pediatr Res. 2012;72(3):277- 284. [14] Schira J, Gasis M, Estrada V, et al.Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain. 2012;135(Pt 2): 431-446.[15] Hu SL, Luo HS, Li JT, et al. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit Care Med. 2010;38(11):2181-2189. [16] Matsuse D, Kitada M, Kohama M,et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration.J Neuropathol Exp Neurol. 2010;69(9):973-985.[17] Pang KM, Sung MA, Alrash-dan MS, et al. Trans-plantation of mesenchymal stem cells from human umbilical cord versus human umbilical cord blood for peripheral nerve regenera-tion. Neural Regen Res. 2010;5(11):838-845.[18] Seo Y, Yang SR, Jee MK, et al.Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann Pick type C1 mice. Cell Transplant. 2011;20(7):1033-1047. [19] Chang YK, Chen MH, Chiang YH, et al.Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells.J Biomed Sci. 2011 Aug 8;18:54.[20] Jones J, Jaramillo-Merchán J, Bueno C, et al. Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol Dis. 2010; 40(2):415-423. [21] Jones J, Jaramillo-Merchán J, Bueno C, et al. Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia Neurobiol Dis. 2010; 40(2):415-423. [22] Dey R, Kemp K, Gray E, et al.Human Mesenchymal Stem Cells Increase Anti-oxidant Defences in Cells Derived from Patients with Friedreich's Ataxia. Cerebellum. 2012 Jul 24. [23] Kemp K, Mallam E, Hares K, et al.Mesenchymal stem cells restore frataxin expression and increase hydrogen peroxide scavenging enzymes in Friedreich ataxia fibroblasts. PLoS One. 2011;6(10):e26098. [24] Yang XF, Xu YF, Lü NW, et al. Umbilical cord mesenchymal stem cell transplantation for the treatment of Duchenne muscular dystrophy. Neural Regen Res. 2011;6(10): 785-789.[25] Dongmei H, Jing L, Mei X, et al. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy. 2011;13(8):913-917.[26] Trouillas P,Takayanagi T,Hallett M,et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J NeurolSci 1997;145:205–211.[27] Matos CA, de Macedo-Ribeiro S, Carvalho AL. C Polyglutamine diseases: the special case of ataxin-3 and Machado-Joseph disease. Prog Neurobiol. 2011;95(1):26-48. [28] Dalous J, Larghero J, Baud O.Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives.Pediatr Res. 2012;71(4 Pt 2):482-490.[29] Taghizadeh RR, Cetrulo KJ, Cetrulo CL.Wharton's Jelly stem cells: future clinical applications.Placenta. 2011;32 Suppl 4: S311-315. [30] Fan CG, Zhang QJ, Zhou JR.Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord.Stem Cell Rev. 2011;7(1):195-207.[31] Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67(1):1-8. [32] Momin EN, Mohyeldin A, Zaidi HA, et al. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther. 2010;5(4):326-344. [33] Zhang MJ, Sun JJ, Qian L, et al.Human umbilical mesenchymal stem cells enhance the expression of neurotrophic factors and protect ataxic mice. Brain Res. 2011; 1402:122-131. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [3] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [4] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [5] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [6] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [7] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [8] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [9] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [10] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [11] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [12] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [13] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [14] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [15] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||