Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (26): 4243-4248.doi: 10.3969/j.issn.2095-4344.1367
Previous Articles Next Articles
Research progress and clinical application of nano-fat
- The Fifth Affiliated Hospital of Guangxi Medical University & The First People’s Hospital of Nanning, Nanning 530000, Guangxi Zhuang Autonomous Region, China
-
Received:2019-04-22 -
Contact:Li Hongmian, MD, Chief physician, The Fifth Affiliated Hospital of Guangxi Medical University & The First People’s Hospital of Nanning, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
About author:Yi Xiaolin, Master candidate, The Fifth Affiliated Hospital of Guangxi Medical University & The First People’s Hospital of Nanning, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81560316, 81760346 (to LHM), 81860341 (to LZJ); the Natural Science Foundation of Guangxi Zhuang Autonomous Region, No. 2016GXNSFDA380016 (to LHM); the Natural Science Foundation of Guangxi Zhuang Autonomous Region (General Program), No. 2018GXNSFAA281148 (to LHM); Nanning Science and Technology Development Plan Project, No. 20173021-2,20183037-1 (to LHM); Guangxi Graduate Education Innovation Program, No. YCBZ2018041 (to LZJ); Nanning Youth Science and Technology Innovation and Entrepreneur Talents Cultivation Project, No. RC20180201 (to LZJ); Guangxi Medical and Health Self-financing Plan Development, No. Z20180679 (to LZJ)
CLC Number:
Cite this article
Yi Xiaolin, Liang Zhijie, Li Hongmian. Research progress and clinical application of nano-fat[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(26): 4243-4248.
share this article
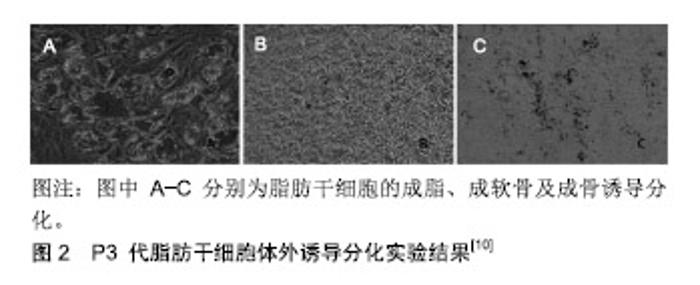
2.1 纳米脂肪的制备 目前制备纳米脂肪的方法有多种,但都是在Tonnard制备方法的基础上进行改进的。Tonnard是用含1 mm的锐利侧孔多端口直径3 mm套管,采用标准抽脂装置从患者腹部或者大腿进行高负压抽脂,将所抽的脂肪是用生理盐水冲洗过滤,再将过滤后的脂肪转移到2个10 mm的注射器内并用连接管连接,来回推送约30来回后可见颗粒脂肪变成了乳糜状,最后在直径为0.6 mm的尼龙纱布上再过滤1次,将收集的纳米脂肪用27 G针头进行皮内注射[6]。在Tonnard的基础上,有学者认为用Tonnard方法处理纳米脂肪会导致脂肪干细胞活力显著降低[7],并提出了更加温和的纳米脂肪处理方法,将处理的脂肪称为vivo纳米脂肪,具体操作方法如下:先用1 mL 0.2 g/L的脂肪酶Ⅰ消化、洗涤,放在一个20 mL的容器内37 ℃孵化15 min,然后以300×g离心7 min,最后再用0.6 mm孔径的滤网过滤。Palluan等[8]也提出不同的制备方法:在Tonnard的方法基础上加入2步离心处理,并将获得的浓缩纳米脂肪称为lipoconcentrate。虽然方法不同,但都是为了提高干细胞的含量。目前制备纳米脂肪的方法还没有统一标准,如何获得脂肪干细胞浓度和活性更高的纳米脂肪需要更多的研究,以开发出更好、更方便的制备方法。 2.2 纳米脂肪的成分和功能 纳米脂肪的具体成分还未明确,但大多数学者研究皆支持其主要主要成分为脂肪干细胞,而成熟的脂肪细胞基本都被破坏[3,6]。脂肪干细胞具有创伤修复功能[9],并具有多向分化能力(在一定条件下可分化为脂肪细胞、成骨细胞、软骨细 胞[10])和自我更新能力[3,11-12],见图2。研究发现脂肪干细胞还具有旁分泌功能,可分泌多种生长因子如血管内皮生长因子、碱性成纤维细胞生长因子等[3],见表1。有研究认为血管内皮生长因子和碱性成纤维细胞生长因子是在脂肪移植中发挥着关键作用的细胞因 子[16-20],血管内皮生长因子主要是与机体内血管内皮生长因子受体2相结合,促进血管内皮细胞增殖及细胞迁移,由此诱导血管的再生,并增加血管的通透性,促进新生血管网的重建,使移植物得到更充足的血供,提高移植物的存活率,并降低移植物的吸收率[21-24];碱性成纤维细胞生长因子则不仅有助于血管形成和再生[25],还可活化各种修复细胞,可作用于内皮细胞趋化因子,也可同时促进内皮细胞的分裂,因此具有超强的促进脂肪细胞增殖分化及血管形成作用[26],在脂肪移植早期可为移植物提供良好的血供,缩短组织缺血时间,并且在移植后期有助于减少移植物的吸收率,提高脂肪移植物存活率[27-28]。此外,其他的因子如炎性相关因子(干扰素γ、白介素和肿瘤坏死因子等[13])均对移植物的存活起到了正向促进作用。 2.3 纳米脂肪的基础研究及临床应用 纳米脂肪成分复杂,具体成分还待研究,其主要发挥作用的成分目前也无定论,但大多数学者支持脂肪干细胞发挥其主要作用,具体如上述。纳米脂肪目前主要应用于组织修复及再生和抗衰老中。 2.3.1 纳米脂肪在软组织填充中的应用 目前市场上常见的填充材料主要有自体脂肪、人工材料、玻尿酸等,由于人工材料属于人体异物,可产生免疫排斥反应和过敏反应,且形态不自然,很多爱美人士无法接受;玻尿酸常用于面部填充,虽然效果快而明显,但效果不持久,且有栓塞血管造成失明等风险;近年来自体脂肪移植因其来源于自身、取材方便、来源充足且可多次使用、无免疫排斥和过敏反应,备受人们的青睐,也是整形美容领域的研究热点。单纯的自体脂肪颗粒注射移植后脂肪存活率不高,Coleman[29]提出移植脂肪存活的关键是移植脂肪和受体组织之间的接触表面积最大化;纳米脂肪经过一系列处理加工后呈乳糜状,不仅保留了脂肪干细胞,而且其体积小,可通过极细的针头(有学者使用过27 G针头用来皮内注射[6]),填充时与周围组织接触面积大,这有益于提高移植物的存活率。Yu等[30]通过裸鼠实验发现,纳米脂肪混合颗粒性脂肪移植12周后移植物存活率明显提高,且移植物内血管化程度明显优于对照组。作者所在团队前期研究将纳米脂肪混和颗粒性脂肪填充与玻尿酸注射填充面部效果进行对比,发现纳米脂肪填充注射后面部软组织缺损及皮肤质地均有较大改善[31]。目前对于纳米脂肪在软组织缺陷填充中的应用和研究较少,其提高移植脂肪生存率的确切效果及与传统脂肪干细胞辅助移植效果的对比仍有待于进一步研究。 2.3.2 纳米脂肪在抗衰老中的应用 衰老是自然现象,随着年龄的增长,皱纹会增多,色素沉着、头发稀疏变白,这是目前人类是无法抗拒的,但可通过一定的方法延缓衰老的进程。既往研究发现,传统的颗粒性脂肪移植术后受区表面皮肤质地能够得到良好改善[32-34],考虑是移植物中脂肪干细胞发挥的作用。但自体颗粒脂肪更适用于容量填充,如乳房、颞部及额部等的大范围填充,而眼睑、嘴唇、皱纹等精细部位的年轻化要求注射针较细,填充物体积也不能过大,故而难以使用传统的颗粒脂肪进行该类型的抗衰老治疗。近年来纳米脂肪在这方面越来越引人注目。 Tonnard用23 G针头将4 mL细颗粒脂肪和6 mL纳米脂肪同时注入到1例61岁女性患者的上唇和下唇皮肤内进行填充,7个月后口周皮肤质量明显改善[6]。Mesguich等[35]对纳米脂肪也有所研究,将获得的纳米脂肪用小针头对4例50-59岁的老年患者口周皱纹行皮内注射,4个月后发现口周皱纹明显改善,患者满意度较高。Uyulmaz等[36]将制备的纳米脂肪在52例患者的瘢痕、皱纹或皮肤变色处行纳米脂肪皮内注射或直接注入瘢痕组织中,治疗前后拍照对比,随访(155±49) d,根据评分系统对皮肤质量进行评估,并记录患者满意度。结果发现有效治疗瘢痕40例(76%)、皱纹6例、皮肤变色6例,治疗后的临床评价显示瘢痕质量明显改善,患者满意度高。提示纳米脂肪移植有助于改善瘢痕、皱纹和皮肤变色。Martin等[37]对毛发较多的区域做了脂肪填充和纳米脂肪移植注射,发现在面部进行脂肪填充很容易,但在头皮区域较困难;而纳米脂肪注射在这些区域则特别方便,特别是在Merkel’s间隙和毛发多的区域需要高精度注射。因此他们认为和脂肪移植相比,纳米脂肪注射在毛发较多的区域(如头皮区)极其方便;若行毛发移植和脱发方面治疗,建议行脂肪移植+纳米脂肪联合注射治疗。Xu等[38]从人体抽出脂肪并加工制备脂肪干细胞和纳米脂肪,将6只BALB/c裸小鼠作为对照组,不作处理任其自然衰老;利用紫外线B辐射18只BALB/c裸小鼠上建立光老化皮肤模型,分为3组处理:分别将PBS (200 μL)、脂肪干细胞(1×106/200 μL)、纳米脂肪(200 μL)注射于小鼠背侧皮肤,4周后采集皮肤标本,通过一般观察评价各组皮肤质地;组织学分析皮肤结构、真皮厚度、胶原纤维排列、毛细血管密度和细胞增殖情况。结果发现,PBS组、脂肪干细胞组、纳米脂肪组皮肤肉眼观察无明显差异;组织学分析显示,脂肪干细胞组和纳米脂肪组真皮明显较PBS组厚(P < 0.05);与PBS组相比,脂肪干细胞和纳米脂肪组皮肤中抗CD31染色的毛细血管更多(P < 0.05);抗ki-67染色观察显示各组真皮平均增殖指数无明显差异;然而与PBS组相比,脂肪干细胞和纳米脂肪组的表皮增殖指数增加(P < 0.05),由此得出纳米脂肪可增加皮肤的真皮层厚度并促进新生血管形成。陈政军等[39]将114例行矫治面部老化手术患者随机分为对照组和观察组,每组57例,对照组单纯给予线雕术治疗,观察组则给予纳米脂肪移植联合线雕治理自傲,结果发现观察组面部改善优良率明显高于对照组(P < 0.05),说明要有效改善面部老化状况、减低并发症等,可考虑选择纳米脂肪移植联合线雕治疗。梁志生等[40]对10例女性患者(面颊部皮肤老化1例,眼周皮肤老化5例,额部皮肤老化2例,瘢痕2例)常规行吸脂术,将获取的脂肪经离心、机械乳化过滤后留取滤过脂肪乳化液,再用27号针头行真皮深层注射,直到皮肤颜色发生黄染变化为止;注射后按疗程定期随访、拍照及接受后续治疗。结果发现10例患者中有效9例(皮肤老化明显改善7例)、无效1例,无严重并发症发生,得出纳米脂肪移植有助于改善面部皮肤质地。以上多位学者的研究结果均表明纳米脂肪注射直接或间接的抗衰老作用显著。 2.3.3 纳米脂肪注射在创面、瘢痕、腺体修复中的应用 常导致瘢痕的原因有烧伤、创伤、手术等,目前临床上治疗瘢痕的方法主要包括皮肤磨削技术、光电治疗技术、皮瓣移植或植皮手术、组织填充技术等。自体脂肪填充因其取材方便、操作简单、不增加供区新的瘢痕、效果明显、可同时改善肤质等优点,目前在临床上应用越来越广泛。之前也有文献报道通过自体脂肪填充,瘢痕外观和局部微循环可得到明显改善[41],在一定情况下甚至可替代上述其他的治疗方法[42]。由于瘢痕组织含纤维化组织多,质地较硬,行瘢痕内注射脂肪要求注射针头要足够细,纳米脂肪因其颗粒直径小,经处理后可顺利通过27 G针头,注入后与瘢痕接触面积相对较大,故纳米脂肪在治疗瘢痕方面有独特的优势。 Bhooshan等[43]应用纳米脂肪进行瘢痕内局部注射治疗,结果证明纳米脂肪瘢痕注射可有效改善瘢痕特征和症状,有助于瘢痕修复。Uyulmaz等[36]在52例患者的瘢痕、皱纹或皮肤变色处行纳米脂肪皮内注射或瘢痕组织直接注入,治疗前后随访(155±49)d,根据评分系统对皮肤质量进行评估,并记录患者满意度,发现有效治疗瘢痕40例(76%)、皱纹6例、皮肤变色6例,治疗后的瘢痕质量明显改善,患者满意度高,结果证明纳米脂肪移植有助于改善瘢痕、皱纹和皮肤变色。Gu等[44]使用浓缩纳米脂肪结合脂肪移植技术治疗面部萎缩性瘢痕,结果证明纳米脂肪结合脂肪移植技术是治疗面部萎缩性瘢痕的安全、有效方法。Tenna等[45]将纳米脂肪皮下注射,结果发现纳米脂肪治疗可有效改善萎缩性瘢痕。Kemalo?lu等[46]对较大创面进行刃厚皮片移植后效果不理想,采用刃厚皮下+纳米脂肪联合治疗后随访6个月,发现效果明显,植皮处柔韧性良好。乳腺修复方面尤其是乳腺癌根治术后造成的双侧乳房不对称、瘢痕挛缩、乳头偏离等,近年来应用自体颗粒脂肪移植或联合背阔肌皮瓣、假体等进行乳房重建较多见,自体脂肪能够在难以修复的象限内或凹陷处进行填充,特别是在乳腺内上象限,这让重建乳房外观更接近自然[47-49]。纳米脂肪在乳腺修复方面的报道较少,但在临床工作中有不少医师逐渐开展自体颗粒脂肪联合纳米脂肪丰乳。王沛森[50]对乳房硅胶假体取出同期行自体脂肪移植隆胸的患者,在乳房假体取出之后进行自体颗粒脂肪和纳米脂肪各层次注射治疗,最终乳房体积得到恢复,乳房形态得以重塑,乳房皮肤、乳头乳晕呈现年轻化的转化,患者较满意,达到临床治愈标准。虽然上述多位学者研究纳米脂肪对瘢痕及创面治疗效果明显,但也只是小样本研究,且具体作用机制尚未明确,所以纳米脂肪对瘢痕作用机制的研究仍需更加深入、更大、更多的样本及数据支撑。 2.3.4 纳米脂肪注射在关节疾病方面的应用 近年来纳米脂肪注射法在关节疾病方面也得到了应用。Mahmmood等[51]将纳米脂肪应用在颞颌关节病上,选择11例被诊断为颞颌关节病的患者(3男8女),年龄在18-34岁之间,其中3例行单侧关节腔注射,8例行双侧关节腔注射,方法如下:将获取的脂肪进行机械乳化,与Tonnard乳化方法不同的是其在2个用转接器链接的 5 mL注射器来回推送50次,将制备好的纳米脂肪用 5 mL注射器18 G针头注入2 mL纳米脂肪于颞下颌关节腔中。治疗后结果显示,患者疼痛程度降低和最大张口度提高,提示纳米脂肪注射法治疗颞颌关节病有疗效、安全、简单,患者可接受且无明显不良反应。 陈鹏等[52]在关节软骨缺损方面进行了动物实验。实验从2周龄大鼠腹股沟部获取脂肪组织,采用机械分离法制备富含血管基质成分的纳米脂肪,在36只8周龄大鼠右侧膝关节股骨滑车中央处建立直径2 mm全层软骨缺损模型,并随机分为3组:空白对照组缺损处旷置;纤维蛋白胶组缺损处植入纤维蛋白胶;血管基质成分+纤维蛋白胶组缺损处植入血管基质成分+纤维蛋白胶。术后6,12周取材,参考国际软骨修复协会软骨修复大体评分标准评价标本修复情况;标本切片后进行苏木精-伊红染色、天狼猩红染色,评价标本修复情况。结果显示:血管基质成分+纤维蛋白胶组软骨修复效果明显优于其他两组(P < 0.05),大体评分高于其他两组(P < 0.05);组织学分析显示,血管基质成分+纤维蛋白胶组缺损区修复组织以透明软骨为主,空白对照组和纤维蛋白胶组缺损区修复组织以纤维组织为主,血管基质成分+纤维蛋白胶组组织学评分高于其他两组(P < 0.05)。结果表明采用机械分离法制备的脂肪来源血管基质混合物,可用于修复大鼠膝关节软骨缺损,是一种较有应用前景的修复关节软骨缺损的方法。纳米脂肪应用与关节疾病方面的研究还较少,且作用机制同样未明确,缺乏大样本数据支撑,需进一步加深该领域的研究。 2.4 纳米脂肪来源脂肪干细胞与酶消化获得脂肪干细胞优缺点对比 酶消化法获得脂肪干细胞的做法是将从患者大腿或者腹部抽取的颗粒脂肪分为2部分,一部分与传统颗粒脂肪处理过程一样,而另一部分需要经过清洗、酶消化、离心等处理获取富含脂肪干细胞的血管基质成分胶,将其与颗粒脂肪细胞混合后共同注射到受区。Tonnard提取的纳米脂肪则主要使用机械乳糜化后过滤得到富含脂肪干细胞的血管基质成分胶,步骤较简洁,并且其经过机械乳糜化后直径较小,可用细针头做及时移植注射,甚至可行皮内、皱纹、瘢痕内注射。有研究证实此方法获得的纳米脂肪中含脂肪干细胞是酶消化法获得的2/3,其他成分无明显差别[6]。由以上可得出,纳米脂肪获取步骤较简洁、体积较小,等量的脂肪细胞通过酶消化法方法获取的脂肪干细胞含量较多。但纳米脂肪不能进行大容量填充。若需大容量填充,两者结合可能是一个不错的选择。 "
| [1]Uzbas F, May ID, Parisi AM, et al. Molecular Physiognomies and Applications of Adipose-Derived Stem Cells.Stem Cell Rev Rep. 2015; 11(2):298-308.[2]Zuk P,Zhu M,Mizuno H,et al.Multilineage cells from human adipose tissue: implications for cell-based therapies.Tissue Eng.2001;7(2):211-228.[3]Liang ZJ,Lu X,Li DQ,et al.Precise Intradermal Injection of Nanofat-Derived Stromal Cells Combined with Platelet-Rich Fibrin Improves the Efficacy of Facial Skin Rejuvenation.Cell Physiol Biochem.2018;47(1):316-329.[4]Yoshimura K,Sato K,Aoi N,et al.Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells.Aesthetic Plast Surg.2008;32(1):48-55.[5]Castro-Govea Y,Garza-Pineda ODL,Lara-Arias J,et al.Cell-Assisted Lipotransfer for the Treatment of Parry-Romberg Syndrome.Arch Plast Surg.2012;39(6):659-662.[6]Tonnard P,Verpaele A,Peeters G,et al.Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg.2013;132(4):1017-1026.[7]Bi HS,Zhang C,Nie FF,et al.Basic and Clinical Evidence of an Alternative Method to Produce Vivo Nanofat.Chin Med J (Engl).2018;131(5):588-593.[8]Pallua N,Grasys J,Kim BS.Enhancement of Progenitor Cells by Two Step Centrifugation of Emulsified Lipoaspirates.Plast Reconstr Surg. 2018; 142(1):1.[9]Luiz C,Christina MT,Radovan B,et al.Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells].Plast Reconstr Surg. 2015; 135(4):999-1009.[10]王倩,汤文燕,汪兆艳,等.不同代数人脂肪干细胞分泌多种细胞因子水平的比较[J].中国组织工程研究,2018,22(9):1319-1324.[11]Yang C,Hu D,Zheng Z,et al.Effects of adipose-derived mesenchymal stem cells over-expressing glial cell line-derived neurotrophic factor on electrically injured sciatic nerve of rats]. Zhonghua Shao shang Zazhi. 2015;31(3):199.[12]Pan W,Zheng C,Jia S,et al.Synergistic enhancement of bone regeneration by LMP-1 and HIF-1α delivered by adipose derived stem cells.Biotechnol Lett.2016;38(3):377-384.[13]Blaber SP, Webster RA, Hill CJ, et al. Analysis of in vitro secretion profiles from adipose-derived cell populations.J Transl Med.2012;10:172.[14]Pu LL.Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells.Plast Reconstr Surg. 2009;124(5):1447-1449.[15]蒋爱梅,王艳梅,董毅龙,等.脂肪干细胞结合碱性成纤维细胞生长因子辅助颗粒脂肪移植的实验研究[J]. 昆明医科大学学报, 2013,34(4):8-12.[16]Yun SP, Min YL, Ryu JM,et al.Role of HIF-1α and VEGF in human mesenchymal stem cell proliferation by 17β-estradiol: involvement of PKC, PI3K/Akt, and MAPKs. Am J Physiol Cell Physiol.2009;296(2):C317.[17]Gu A,Tsark W,Holmes KV,et al.Role of Ceacam1 in VEGF induced vasculogenesis of murine embryonic stem cell-derived embryoid bodies in 3D culture.Exp Cell Res.2009;315(10):1668-1682.[18]Hiranmoy D,George JC,Matthew J,et al.Stem cell therapy with overexpressed VEGF and PDGF genes improves cardiac function in a rat infarct model.PLoS One.2009;4(10):e7325.[19]David Z,Arsalan S,Gen S,et al.Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair.Biochem Biophys Res Commun. 2009; 390(3):834-838.[20]Guo X,Lian R,Guo Y,et al.bFGF and Activin A function to promote survival and proliferation of single iPS cells in conditioned half-exchange mTeSR1 medium.Human Cell.2015;28(3):122.[21]An SS,Jin HL,Kim KN,et al.Neuroprotective effect of combined hypoxia-induced VEGF and bone marrow-derived mesenchymal stem cell treatment.Childs Nerv Syst.2010;26(3):323-331.[22]Sun J,Sha B, Zhou W, et al.VEGF-mediated angiogenesis stimulates neural stem cell proliferation and differentiation in the premature brain.Biochem Biophys Res Commun.2010;394(1):146-152.[23]Lee YB,Polio S,Lee W,et al.Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture.Exp Neurol. 2010;223(2): 645-652.[24]Deng ZG1,Li B,Zu C.The Effect of Allogenic Hematopoietic Stem Cell Transplantation on Tumor Recurrence and Metastasis of Hepatocellular Carcinoma after Hepatectomy and the Relationship with Presence of AFP mRNA and VEGF-C mRNA in Peripheral Blood.Sichuan Da Xue Xue Bao Yi Xue Ban.2010;41(2):256-260.[25]Xie JX,Feng Y,Yuan JM,et al.Positive effects of bFGF modified rat amniotic epithelial cells transplantation on transected rat optic nerve. PLoS One. 2014; 10(3):e119119.[26]Yujuan T,Shaoquan C,Zaizhong Z,et al.[Effect of propranolol gel on plasma VEGF, bFGF and MMP-9 in proliferating infantile hemangiomas of superficial type].Zhonghua Zheng Xing Wai Ke Za Zhi. 2015;31(4): 268-273.[27]Xu P, Yu Q, Huang H, et al. Nanofat Increases Dermis Thickness and Neovascularization in Photoaged Nude Mouse Skin.Aesthetic Plast Surg.2018;42(2):343-351.[28]Yu Q,Cai Y,Huang H,et al.Co-Transplantation of Nanofat Enhances Neovascularization and Fat Graft Survival in Nude Mice.Aesthet Surg J.2018;38(6):667-675. [29]Coleman SR.Structural fat grafting: more than a permanent filler.Plast Reconstr Surg.2006;118(Suppl):108S-120S.[30]Yu Q,Cai Y,Huang H,et al. Co-Transplantation of Nanofat Enhances Neovascularization and Fat Graft Survival in Nude Mice.Aesthet Surg J. 2018;38(6):667-675. [31]Wei H, Gu SX, Liang YD,et al. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget. 2017;8(40): 68542-68556.[32]Petersen GF,Hilbert BJ,Trope GD,et al.Direct Conversion of Equine Adipose-Derived Stem Cells into Induced Neuronal Cells Is Enhanced in Three-Dimensional Culture.Cell Reprogram.2015;17(6):419.[33]Sheykhhasan M,Qomi RT,Ghiasi M.Fibrin Scaffolds Designing in order to Human Adipose-derived Mesenchymal Stem Cells Differentiation to Chondrocytes in the Presence of TGF-β3.Int J Stem Cell. 2015;8(2): 219-227.[34]Mehrabani D,Babazadeh M,Tanideh N,et al.The Healing Effect of Adipose-Derived Mesenchymal Stem Cells in Full-thickness Femoral Articular Cartilage Defects of Rabbit.Int J Organ Transplant Med. 2015; 6(4):165-175.[35]Mesguich BF,Bertrand B,Magalon J,et al. Treatment of wrinkles of the upper lip by emulsified fat or "Nanofat": Biological and clinical study about 4Â cases].Ann Chir Plast Esthet.2018;63(1):31-40. [36]Uyulmaz S,Macedo NS,Rezaeian F,et al.Nanofat Grafting for Scar Treatment and Skin Quality Improvement.Aesthet Surg J. 2018;38(4): 421-428. [37]Martin A,Maladry D,Esmaeli A,et al.Fat grafting of hairy areas of head and neck - comparison between lipofilling and nanofat grafting procedures in a cadaveric study.J Stomatol Oral Maxillofac Surg. 2018;119(4):274-278.[38]Xu P, Yu Q, Huang H, et al. Nanofat Increases Dermis Thickness and Neovascularization in Photoaged Nude Mouse Skin.Aesthetic Plast Surg. 2018;42(2):343-351.[39]陈政军,陈芸,牟方国,等.颜面部纳米脂肪移植联合线雕在矫治面部老化中的应用[J].中国医疗美容,2018,8(7):4-7.[40]梁志生,杨时昕,张华彬,等.Nanofat在面部非结构性移植中的临床观察[J].中国美容整形外科杂志,2015,26(5):279-281.[41]Pallua N,Baroncini A,Alharbi Z,et al.Improvement of facial scar appearance and microcirculation by autologous lipofilling.J Plast Reconstr Aesthet Surg. 2014;67(8):1033-1037. [42]Eva G,Joan F,Guillermo R.Autologous fat grafting for correction of unaesthetic scars.Ann Plast Surg.2012;69(5):550-554.[43]Bhooshan LS,Devi MG,Aniraj R,et al.Autologous emulsified fat injection for rejuvenation of scars: A prospective observational study. Indian J Plast Surg.2018;51(1):77-83.[44]Gu Z,Li Y,Li H.Use of Condensed Nanofat Combined With Fat Grafts to Treat Atrophic Scars. JAMA Facial Plast Surg.2018;20(2):128-135.[45]Tenna S,Cogliandro A,Barone M,et al.Comparative Study Using Autologous Fat Grafts Plus Platelet-Rich Plasma With or Without Fractional CO2 Laser Resurfacing in Treatment of Acne Scars: Analysis of Outcomes and Satisfaction With FACE-Q.Aesthetic Plast Surg. 2017; 41(3):661-666.[46]Kemalo?lu CA.Nanofat grafting under a split-thickness skin graft for problematic wound management. Springerplus.2016;5:138.[47]Economides JM,Song DH.Latissimus Dorsi and Immediate Fat Transfer (LIFT) for Complete Autologous Breast Reconstruction.Plast Reconstr Surg. 2018;6(1):1.[48]Biazus JV,Falcão CC,Parizotto AC,et al.Immediate Reconstruction with Autologous fat Transfer Following Breast-Conserving Surgery.Breast J. 2015;21(3):268-275.[49]Moltó GR, González AV,Villaverde Doménech ME.Fat grafting in immediate breast reconstruction. Avoiding breast sequelae.Breast Cancer. 2016; 23(1):134-140.[50]王沛森.乳房硅胶假体取出同期自体颗粒脂肪移植丰乳[Z].[51]Mahmmood VH,Shihab SM.Assessment of Therapeutic Effect of Intra- Articular Nanofat Injection for Temporomandibular Disorders.J Craniofac Surg.2018.doi: 10.1097/SCS.0000000000004938. [Epub ahead of print][52]陈鹏,黄绍代,鲁长风,等.机械分离法制备脂肪来源血管基质混合物修复大鼠膝关节软骨缺损[J].中国矫形外科杂志, 2018,26(16):1504-1507. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [3] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [4] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [5] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [6] | Shu Wenbo, Chen Mengchi, Li Hua, Huang Liqian, Huang Binbin, Zhang Wenhai, Wu Yachen, Wang Zefeng, Li Qiaoli, Liu Peng. Correlation between body fat distribution and characteristics of daily physical activity in college students [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1277-1283. |
| [7] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [8] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [9] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [10] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [11] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [12] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [13] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [14] | Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling. Photoreceptor cell replacement therapy for retinal degeneration diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1116-1121. |
| [15] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

