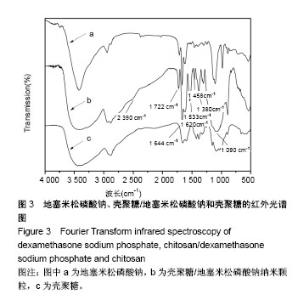
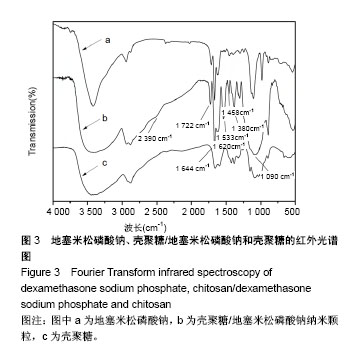
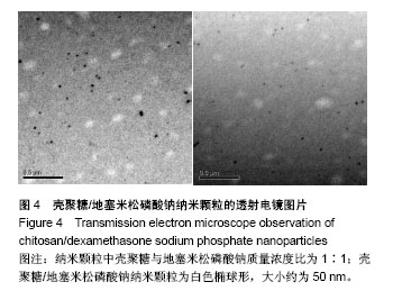
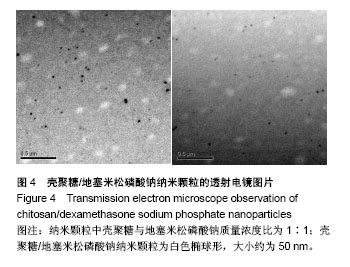
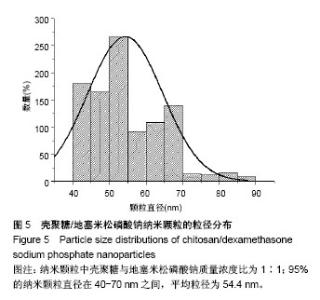
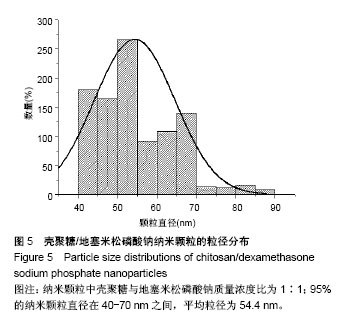
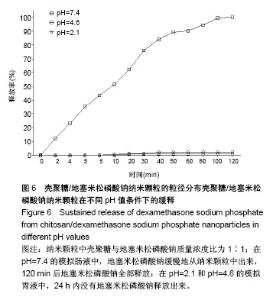
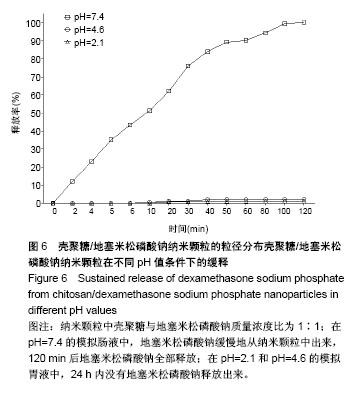
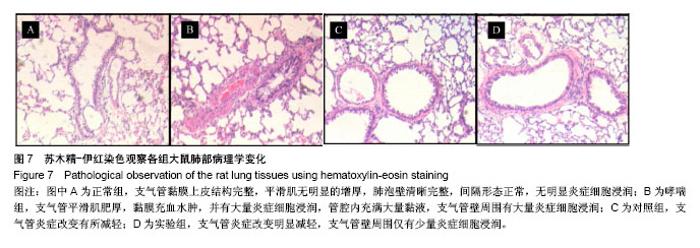
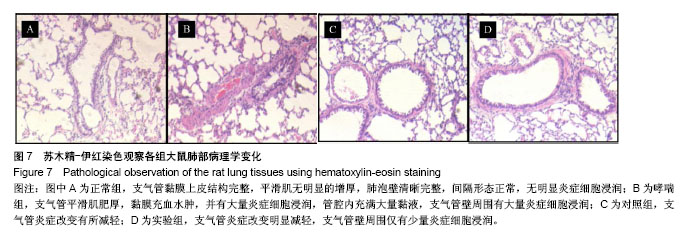
| [1] 谈增.膝关节注射用地塞米松透明质酸凝胶的制备与研究[D].杭州:浙江工业大学,2015.[2] 刘庆清.组蛋白去乙酰化酶在哮喘小鼠气道的表达及维生素D和地塞米松对其影响的研究[D].泸州: 西南医科大学,2017.[3] 施雪涛.骨修复药物控释微球支架的多级构建及干细胞介导分化研究[D].广州:华南理工大学,2010.[4] Li R,Si JJ,Tang P.Enhancement of Electrostatic Charge Dissipation Properties of Polymers By a Sustained‐release Effect of Mesoporous Silica Nanoparticles.Polym Adv Technol. 2016;27(5):615-622.[5] Follmann HDM,Oliveira ON,Lazarin-Bidóia D,et al. Multifunctional hybrid aerogels: hyperbranched polymer-trapped mesoporous silica nanoparticles for sustained and prolonged drug release. Nanoscale.2018;10(4):1704-1715.[6] 崔菁,张亚彬,马思琪,等.拉胶/聚乙烯醇复合材料的制备及生物相容性评价[J].中国组织工程研究, 2017,21(2):215-220.[7] 李瑞端,张建军.聚乳酸-羟基乙酸聚合物载入羟基喜树碱载药纳米粒子的制备及表征[J].化学反应工程与工艺, 2016,32(6): 542-546.[8] 黄妍瑜.生物大分子纳米载药体系的功能化设计及其在肿瘤诊疗中的研究[D].广州:暨南大学,2016.[9] 宋一凡,柴云,张普玉.刺激响应性聚合物载药纳米胶束研究进展[J].化学研究,2016,27(5): 655-659.[10] 董丽艳,李晓华,焦晓雯,等.他克莫司载药胶束的制备与制剂学性质[J].沈阳药科大学学报,2016, 33(9):679-685.[11] 李哲,马海英.醋酸地塞米松脂质体滴眼液的制备[J].中南药学, 2017,15(2):169-174.[12] 隋晓佳,马姝.激素类药物醋酸地塞米松纳米粒的研制及其质量控制[J].吉林医学,2017,38(11): 2111-2112.[13] 刘方刚,孙胜楠,杜鹏飞,等.纳米羟基磷灰石-地塞米松载药体系的制备及体外缓释研究[J].江南大学学报(自然科学版), 2012, 11(3): 326-331.[14] 陈诗琪,张贤明.天然高分子壳聚糖的特性及其应用[J].应用化工, 2016,45(1):152-155.[15] 孙晓婷,郭亚.壳聚糖的应用及发展[J].成都纺织高等专科学校学报, 2016,33(2):165-169.[16] 徐敬,赵建宁,徐海栋,等.壳聚糖及其衍生物在软骨组织工程中的应用[J].中国组织工程研究,2015, 19(25):4081-4085.[17] George M,Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan--a review.J Control Release.2006;114(1):1-14.[18] 李娟,赵清喜.壳聚糖微球包裹幽门螺杆菌全菌蛋白抗原的制备及体外释放性能[J].中国组织工程研究, 2015,19(21):3334-3338.[19] 李益丰,张姝江,陈艺,等.壳聚糖载药微球/大孔磷酸钙骨水泥支架的力学性能研究[J].中华关节外科杂志:电子版, 2016,10(2):38-41.[20] 张春雷,杨天剑,饶华新,等.苦参碱/壳聚糖/羧甲基壳聚糖载药膜的制备及其体外释放行为分析[J]. 南方医科大学学报, 2016, 36(7):1024-1028.[21] 席少晖,黄棣,杜晶晶,等.原位纳米羟基磷灰石/壳聚糖复合载药微球的制备及释放性能研究[J].化工新型材料, 2016,44(8): 212-214.[22] 刘腾辉,吴成飞,赵玉猛,等.壳聚糖载药微胶囊的制备技术及其应用[J].化工管理,2016,29(32): 132-133.[23] 邓广汉.壳聚糖修饰地塞米松脂肪乳滴眼液研究[D].广州:广东药科大学,2016.[24] 韦舜.试论表皮细胞生长因子复合地塞米松及含奥硝唑的壳聚糖膜修复兔口腔溃疡[J].全科口腔医学电子杂志, 2016,3(12):57,59.[25] 谷颖.小儿下呼吸道感染行氨溴索联合地塞米松超声雾化吸入的疗效观察[J].药物与人,2014,27(9):47-47.[26] Wang YH,Yu CM,Pan ZQ,et al.A gold electrode modified with hemoglobin and the chitosan@Fe3O4 nanocomposite particles for direct electrochemistry of hydrogen peroxide.Microchimica Acta. 2013;180(7):659-667.[27] 宋丽敏,李明,倪雅欣,等.高效氯氰菊酯/海藻酸钠/壳聚糖凝胶微球的制备[J].应用化工,2016, 45(11):2118-2120.[28] Pignatello R,Stancampiano AH,Ventura CA,et al. Dexamethasone sodium phosphate-loaded Chitosan based delivery systems for buccal application.J Drug Target.2007; 15(9):603-610.[29] Cázares-delgadillo J,Balaguer-fernández C,Calatayud-pascual A,et al.Transdermal iontophoresis of dexamethasone sodium phosphate in vitro and in vivo: effect of experimental parameters and skin type on drug stability and transport kinetics.Eur J Pharm Biopharm. 2010;75(2): 173-178.[30] Murakami K,Aoki H,Nakamura S,et al.Hydrogel Blends of Chitin/chitosan, Fucoidan and Alginate as Healing-impaired Wound Dressings.Biomaterials.2010;31(1):83-90.[31] 贾利娜,何俊男,赵敬东,等.壳聚糖-海藻酸钠载药微球的缓释性能研究[J].广州化工,2016,44(2): 65-68.[32] Peppas NA.1.Commentary on an Exponential Model for the Analysis of Drug Delivery: Original Research Article: a Simple Equation for Description of Solute Release: I Ii. Fickian and Non-fickian Release From Non-swellable Devices in the Form of Slabs, Spheres, Cylinde.J Control Release. 2014;190: 31-32.[33] 覃志高,罗荣双.醋酸地塞米松剂型研究进展[J].中国合理用药探索, 2010,19(4):82-84.[34] 杨勇博,孙庆申,车小琼,等.壳聚糖在药物剂型中的应用[J].中国组织工程研究,2008,12(41): 8131-8134. |