Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (9): 1338-1343.doi: 10.3969/j.issn.2095-4344.0462
Previous Articles Next Articles
Repair of large-segmental femoral defects using lentivirus-mediated bone morphogenetic protein 2/bone marrow mesenchymal stem cells/demineralized bone matrix
Tao Xuan, Li Qiang, Li Shi-peng, Shi Zheng-song, Zhou Zhen-jie
- Department of Emergency Traumatic Surgery, the Affiliated Hospital of Guilin Medical University, Guilin 541001, Guangxi Zhuang Autonomous Region, China
-
Revised:2017-12-20Online:2018-03-28Published:2018-04-03 -
Contact:Li Qiang, Master, Chief physician, Professor, Master supervisor, Department of Emergency Traumatic Surgery, the Affiliated Hospital of Guilin Medical University, Guilin 541001, Guangxi Zhuang Autonomous Region, China -
About author:Tao Xuan, Master candidate, Department of Emergency Traumatic Surgery, the Affiliated Hospital of Guilin Medical University, Guilin 541001, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 31160199; the Natural Science Foundation of Guangxi Zhuang Autonomous Region, No. 2014GXNSFAA118263; the Natural Science Foundation of Guilin Medical University, No. LX201425
CLC Number:
Cite this article
Tao Xuan, Li Qiang, Li Shi-peng, Shi Zheng-song, Zhou Zhen-jie. Repair of large-segmental femoral defects using lentivirus-mediated bone morphogenetic protein 2/bone marrow mesenchymal stem cells/demineralized bone matrix[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(9): 1338-1343.
share this article
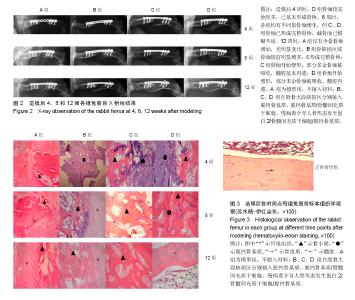
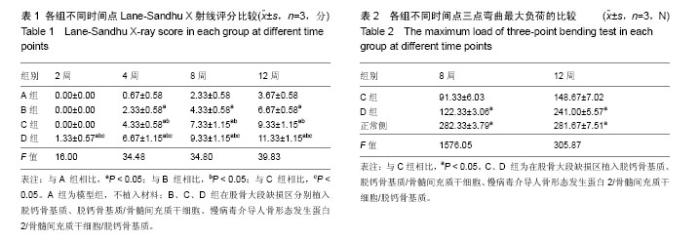
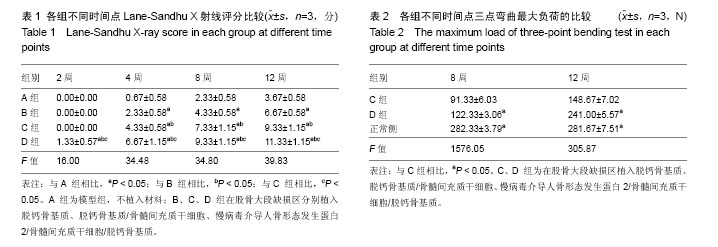
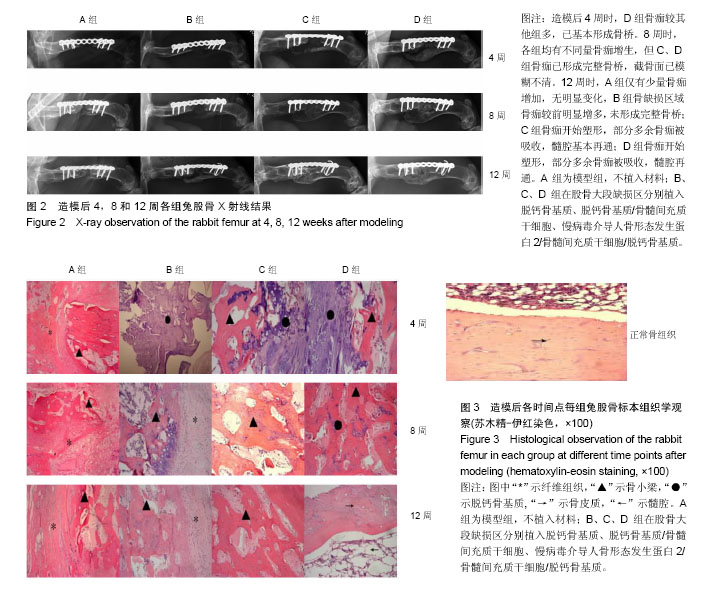
2.1 实验动物数量分析 48只新西兰大白兔均进入结果分析。 2.2 各组兔左侧股骨X射线观察 见图2。各组在各时间点的钢板螺钉固定牢固。 造模后2周时,A、B、C组在骨缺损区域均只有少量骨痂出现,D组新生骨痂较多,未形成完整骨桥。 4周时,A组骨缺损区域近远端新生骨痂已向中间延伸;B组钢板对侧骨痂密度影较术后2周时增高,骨缺损区域也有新生骨痂;C组骨痂已基本形成骨桥,截骨面稍模糊;D组骨痂已基本形成骨桥,截骨面稍模糊。 8周时,A组骨缺损区域近远端新生骨痂继续向中间延伸,近远端新生骨痂未形成连接;B组骨痂量未见明显变化,截骨面较前模糊;C组骨痂已形成完整骨桥,截骨面已模糊不清;D组骨痂已完全长满骨缺损区域,截骨面模糊。 12周时,A组仅有少量骨痂增加,无明显变化,A组骨缺损最终未能完成修复;B组骨缺损区域骨痂较前明显增多,未形成完整骨桥;B组骨缺损最终未能完成修复;C组骨痂开始塑形,部分多余骨痂被吸收,髓腔基本再通;C组骨缺损修复完成;D组骨痂开始塑形,部分多余骨痂被吸收,髓腔再通;D组骨缺损修复完成。 A、B、C、D组4,8,12周的Lane-Sandhu X射线评分均有显著差异(P < 0.05),D组2周的Lane-Sandhu X射线评分与A、B、C组相比有明显差异(P < 0.05),见表1。提示在兔股骨大段缺损修复中慢病毒-hBMP-2/BMSCs/脱钙骨基质 效果优于单纯脱钙骨基质和BMSCs/脱钙骨基质。随时间推移,各组Lane-Sandhu X射线评分依次增高,提示骨缺损的修复有一定的时间依赖性。 2.3 各组兔股骨生物力学测试结果 骨缺损修复后兔股骨形状不规则,无法建立弹性模量计算模型,故实验只能通过三点弯曲试验测量出最大负载压力。修复兔股骨标本中无骨性连接的也无法完成力学测试。8、12周时C、D组组间及同正常股骨三点弯曲最大负荷比较,差异均有显著性意义(P < 0.05),见表2。 2.4 兔股骨标本苏木精-伊红染色结果 见图3。 A组造模后2周见机化血肿及纤维组织,无明显骨小梁形成;4周时见大量纤维组织增生,少量骨小梁形成;8周骨小梁较前有所增多,但仍然被大量的纤维组织填充;12周可见部分排列紊乱的骨小梁和大量的纤维组。 B组造模后2周大量脱钙骨基质骨架,少量机化血肿及纤维组织,无明显骨小梁形成;4周脱钙骨基质骨架已部分降解,周围纤维组织侵入其中,有少许不规则的骨小梁包绕;8周无形态典型脱钙骨基质骨架,纤维组织侵入较前增多,外层排列紊乱的骨小梁增多;12周无脱钙骨基质,可见较多排列紊乱的骨小梁和部分纤维组织。 C组造模后2周大量脱钙骨基质骨架,少量机化血肿及纤维组织,外层已有幼稚骨小梁形成;4周脱钙骨基质骨架已部分降解,骨小梁之间的连接增多;8周脱钙骨基质骨架基本降解,大量骨小梁形成,骨小梁之间连接较前增多;12周骨小梁已基本连接成线,开始塑形为皮质骨,髓腔再通不显著。 D组造模后2周大量脱钙骨基质骨架,少量机化血肿及纤维组织,外层已有大量幼稚骨小梁形成;4周脱钙骨基质骨架已部分降解,大量新生骨小梁出现,骨小梁之间的连接增多并连接成线;8周脱钙骨基质骨架基本降解,骨缺损区域大量骨小梁形成,骨小梁之间连接较前增多;12周已基本形成新的骨皮质,并已有髓腔再同。股骨正常形态可见皮质骨与髓腔分界清晰,骨细胞分布均匀,细胞基质平行排列有序,可见骨皮质中散在分布数个小血管腔隙。"
| [1] Schlickewei CW, Laaff G, Andresen A, ET AL. Bone augmentation using a new injectable bone graft substitute by combining calcium phosphate and bisphosphonate as composite-an animal model. J Orthop Surg Res. 2015;10: 116.[2] Dimitriou R, Mataliotakis GI, Angoules AG, et al. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury.2011;42(5):S3-15.[3] Sims L, Kulyk P, Woo A. Intraoperative culture positive allograft bone and subsequent postoperative infections: a retrospective review. Can J Surg. 2017;60(2):94-100.[4] Hinsenkamp M, Muylle L, Eastlund T, et al. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation Project NOTIFY. Int Orthop. 2012; 36(3):633-641.[5] Mroz TE, Joyce MJ, Lieberman IH, et al. The use of allograft bone in spine surgery: is it safe? Spine. 2009; 9(4): 303-308.[6] Samorezov JE, Alsberg E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv Drug Deliv Rev. 2015; 48:45-67[7] Mini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng.2012;40( 5):363-408.[8] Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol.2012; 30(10): 546-554. [9] Lebouvier A, Poignard A, Cavet M, et al. Development of a simple procedure for the treatment of femoral head osteonecrosis with intra-osseous injection of bone marrow mesenchymal stromal cells: study of their biodistribution in the early time points after injection. Stem Cell Res Ther. 2015; 6(1): 68. [10] May AN, Radhakrishnan V, Dalia A, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2013; 9(1):32.[11] Sekiya I, Larson BL, Vuoristo JT, et al. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res. 2004; 19(2): 256-264.[12] Escors D, Breckpot K, Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp (Warsz).2010;58(2):107-119.[13] Suwanmanee T, Hu G, Gui T, et al. Integration-deficient lentiviral vectors expressing codon-optimized R338L human FIXrestore normal hemostasis in Hemophilia B mice. Mol Ther. 2014;22(3):567-574.[14] 李强,孙正义,严冬雪,等.脱钙骨基质材料的生物学表现:降解性能、孔隙率及其黏附性能特征[J].中国组织工程研究与临床康复,2007,11(31):6112-6124.[15] 陈佳滨,李强,茹嘉,等.腺病毒介导BMP-2 和EGFP基因转染兔骨髓基质干细胞及种植脱钙骨的体外观察[J].中国骨质疏松杂志,2014,20(8):869-874.[16] 李诗鹏,李强,石正松,等.基因重组腺病毒载体与慢病毒载体转染兔骨髓间充质干细胞的对比[J].中国组织工程研究,2017, 12(9): 1340-1345.[17] Baltzer AW, Lattermann C, Whalen J D, et al. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee Surg Sports Traumatol Arthrosc. 1999; 7(3):197-202.[18] Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am.1987; 18(2):123-225.[19] 陈佳滨,武成聪,李强,等.转基因技术及骨髓间充质干细胞在骨缺损修复中的应用与展望[J].临床医学工程,2013, 20(11): 1457-1459.[20] Vo TN, Shah SR, Lu S, et al. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials. 2016; 83:1-11.[21] Cui N, Qian J, Wang J, et al. Preparation, physicochemical properties and biocompatibility of PBLG/PLGA/bioglass composite scaffolds. Mater Sci Eng C Biomim Supramol Syst. 2017;71:118-124.[22] Pot M W, De L K, Van PDK, et al. Unidirectional BMP2-loaded collagen scaffolds induce chondrogenic differentiation. Biomed Mater.2017; 13(1):015007.[23] Peng K T, Hsieh M Y, Lin C T, et al. Treatment of critically sized femoral defects with recombinant BMP-2 delivered by a modified mPEG-PLGA biodegradable thermosensitive hydroge. BMC Musculoskelet Disord. 2016; 17(1):286.[24] Sharma S, Sapkota D, Xue Y, et al. Adenoviral mediated expression of BMP2 by bone marrow stromal cells cultured in 3D copolymer scaffolds enhances bone formation. Plos One. 2016;11(1):e0147507.[25] Krishnan L, Priddy LB, Esancy C, et al. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater. 2017;49: 101-112.[26] Tatara AM, Mikos AG. Tissue engineering in orthopaedics. J Bone Joint Surg Am. 2016; 98(13):1132-1139.[27] Mauffrey C, Barlow BT, Smith W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015;23(3):143-153.[28] 陈思圆,王簕,江汕,等.植入含感觉神经束和血管束组织工程骨修复股骨大段骨缺损:降钙素基因相关肽Ⅰ型受体的中长期表达[J].中国组织工程研究, 2017,12(6):488-853.[29] Kang JW, Suh DH, Park JH, et al. Repair of large segmental bone defect using vascularized small corticocancellous bone in rabbit femur. J Reconstr Microsurg. 2017; 33(9):649-659.[30] Baltzer AW, Lattermann C, Whalen JD, et al. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7(9):734-739. |
| [1] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1602-1608. |
| [2] | Liu Xiaogang, Li Tian, Zhang Duo. Effect and mechanism of the effective components of Chinese medicine on promoting the differentiation of bone marrow mesenchymal stem cells into chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 121-126. |
| [3] | Shuai Zhiqin, Chen Jiameng, Liu Taotao, Hu Anling, Li Lisheng, Yu Limei, Xu Shangfu. Research status and problems of stem cells and their derived exosomes for prevention and treatment of vascular restenosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 138-144. |
| [4] | Li Zhongkang, Zheng Jiahua, Tian Yanpeng, Huang Xianghua. Latest progress and mechanisms of mesenchymal stem cells on premature ovarian failure [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 145-152. |
| [5] | Wang Shuyun, Xie Junhui, Yu Xuefeng. Effect and mechanism of mesenchymal stem cells in the treatment of diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 153-158. |
| [6] | Lin Miaoyuan, Li Yuwan, Liu Yi, Chen Bei, Zhang Li. Research hotspots and application value of tissue-engineered skin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 159-166. |
| [7] | Song Huifang, Tan Jiayin, Kang Yi, Li Bin, Bi Zhifei, Long Nü, Xia Zhongnian, Guo Rui. Hypoxic pretreatment enhances the protective effect of aged human bone marrow mesenchymal stem cells conditioned medium against H9C2 oxidative stress damage [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 1-6. |
| [8] | Xie Xingqi, Hu Wei, Tu Guanjun. Bone marrow mesenchymal stem cells-derived exosomes combined with chondroitinase ABC for treating spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 20-26. |
| [9] | Zhang Xuelei, Luo Gan, Yu Shenghui, Gu Zuchao, Peng Xu, He Xueling, Liu Yan, Zhang Xiaomei. Acellular nerve scaffold combined with bone marrow mesenchymal stem cells and platelet-rich gel for femoral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 27-32. |
| [10] | Zhao Laihe, Xia Bing, Ma Teng, Gao Jianbo, Li Shengyou, Gao Xue, Zheng Yi, Hu Guangwen, Luo Zhuojing, Huang Jinghui. Extracellular matrix of Schwann-like cells induced by bone marrow mesenchymal stem cells promotes axonal regeneration after peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 33-39. |
| [11] | Mai Liping, He Guodong, Chen Shaoxian, Zhu Jiening, Hou Xinghua, Zhang Mengzhen, Li Xiaohong. Expression of aldehyde dehydrogenase 3B1 during human bone marrow mesenchymal stem cells senescence [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 40-44. |
| [12] | Yang Tengyun, Li Yanlin, Liu Dejian, Wang Guoliang, Zheng Zhujun. Chondrogenic differentiation of peripheral blood-derived mesenchymal stem cells induced by transforming growth factor beta 3: a dose-effect relationship [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 45-51. |
| [13] | Huang Tao, Cheng Zhijian, Jia Zhiqiang, Zhao Xiaoguang, Wang Lei, Zhai Wenjing, Zhou Yongxin. Mechanism by which miR-146a regulates osteogenic differentiation of adipose derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 70-75. |
| [14] | Jing Jin, Zhao Shandi, Chen Long, Peng Shuanglin, Tang Hui, Guo Daijin, Zeng Xinyi, Xiao Jingang. Repair of calvarial defects in osteoporotic mice by adipose-derived stem cells combined with biphasic calcium phosphate ceramic scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 90-95. |
| [15] | Cui Shuaishuai, Yang Xiaohong. Effects of miRNA in self-renewal, multidirectional differentiation, fate and function regulation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 101-106. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||