Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (16): 2613-2618.doi: 10.12307/2024.286
Previous Articles Next Articles
Ferroptosis in bone diseases: therapeutic targets of osteoporosis
Xie Heng1, Gu Ye1, Gu Yingchu1, Wu Zerui1, Fang Tao1, Wang Qiufei1, Peng Yuqin1, Geng Dechun2, Xu Yaozeng2
- 1Department of Orthopedics, Changshu First People’s Hospital, Changshu Affiliated Hospital of Soochow University, Changshu 215500, Jiangsu Province, China; 2Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2023-02-23Accepted:2023-04-24Online:2024-06-08Published:2023-07-31 -
Contact:Gu Ye, MD, Associate chief physician, Department of Orthopedics, Changshu First People’s Hospital, Changshu Affiliated Hospital of Soochow University, Changshu 215500, Jiangsu Province, China -
About author:Xie Heng, Master candidate, Department of Orthopedics, Changshu First People’s Hospital, Changshu Affiliated Hospital of Soochow University, Changshu 215500, Jiangsu Province, China -
Supported by:Jiangsu Province Young Medical Key Talents Program, No. QNRC2016751 (to GDC); Jiangsu Six-One Project Funding Project, No. LQY2016033 (to GDC); Key Research & Development Plan (Social Development) Project of Jiangsu Provincial Department of Science and Technology, Nos. BE2021673 and BE2020666 (to GY); Science and Technology Development Plan Project of Suzhou Science and Technology Bureau, Nos. SYSD2022023 and SYSD2020013 (to GY); The Diagnosis and Treatment Project of Clinical Key Diseases of Suzhou Municipal Health and Health Commission, No. LCZX201824 (to GY); Science and Technology Plan Projects of Changshu Health Commission, Nos. CS202119 (to WQF) and CS201817 (to GY); Soochow University Horizontal Project, No. H200833 (to GY)
CLC Number:
Cite this article
Xie Heng, Gu Ye, Gu Yingchu, Wu Zerui, Fang Tao, Wang Qiufei, Peng Yuqin, Geng Dechun, Xu Yaozeng. Ferroptosis in bone diseases: therapeutic targets of osteoporosis[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2613-2618.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
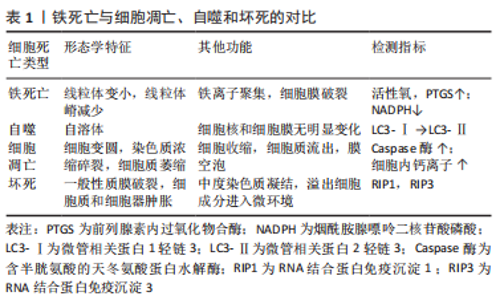
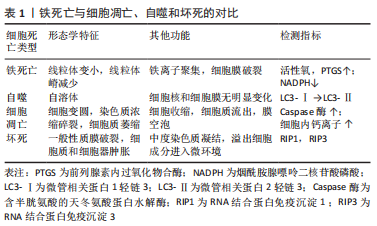
2.1 铁死亡与自噬、坏死、凋亡的区别 铁死亡是一种新的细胞死亡模式,其特征是铁介导的脂质过氧化的积累。2012年,DIXON等[13-14]首次提出了铁死亡的定义,这是一种以脂质活性氧积累为特征的铁依赖性非凋亡细胞死亡模式。铁死亡是一种与凋亡、坏死和自噬不同的铁依赖性新型程序性细胞死亡模式[13]。在细胞形态学特征方面,线粒体萎缩、线粒体危象减少甚至消失、膜密度增加。在生物学特性方面,胱氨酸摄取减少,谷胱甘肽耗竭,胱氨酸/谷氨酸反转运蛋白活性抑制,铁离子和活性氧异常聚集。在基因水平上,铁死亡主要受核糖体蛋白L8、铁反应元件结合蛋白2、酰基CoA合成酶家族成员2以及代谢和储存基因TFRC、ISCU、FTH1、FTL和SLC11A2的调节[15]。而细胞凋亡的特征是核碎裂、凋亡小体的形成以及促凋亡B细胞淋巴瘤2等蛋白的激活。它导致线粒体外膜通透性增加和活性氧的产生,从而释放凋亡因子并促进半胱氨酸级联激活[16-17]。在坏死过程中,质膜破裂、细胞质细胞器肿胀、核小体间DNA断裂缺陷、ATP消耗和损伤相关分子模式释放[18-19]。自噬的特点是自噬液泡的积累、细胞质空泡化、染色质不凝结、微管相关蛋白1轻链3转化为微管相关蛋白2轻链3,p62裂解[20-21],见表1。"
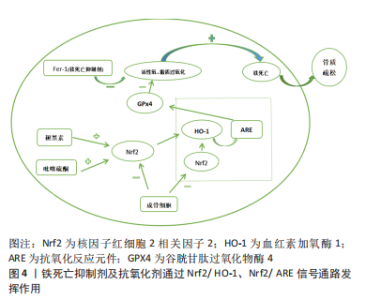
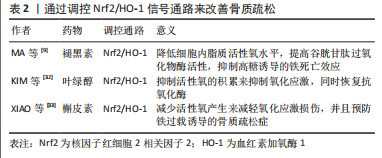
2.2 骨质疏松靶细胞与铁死亡的关系 2.2.1 铁死亡和成骨细胞 铁稳态的存在对细胞的正常生理功能很重要。研究发现,骨质疏松与铁稳态密切相关,细胞内铁稳态主要由多种铁相关蛋白调节,包括细胞内铁蛋白、二价金属转运蛋白1、转铁蛋白受体和转铁蛋白[22]。铁超载是由成骨细胞中二价金属转运蛋白1的过度表达引起的,导致氧化应激反应,并最终影响成骨细胞的成骨功能,这在2型糖尿病性骨质疏松的发病机制中起着重要作用[23-25]。MA等[9]和WANG等[26]都发现,高糖可以通过增加双层线粒体膜的密度和减少线粒体嵴来影响成骨细胞的线粒体形态,并且线粒体形态的这些变化与铁死亡特征一致。高糖还改变成骨功能相关指标,包括骨保护素和骨钙素的表达减少,碱性磷酸酶活性降低,并减少矿化结节的形成[9,26]。此外,高糖还可以增加细胞内活性氧含量,促进过氧化脂的积累,并降低细胞中脱铁相关蛋白谷胱甘肽过氧化物酶4(glutathione peroxidase 4,GPX4)的表达水平,导致成骨细胞的脱铁和成骨功能下降。先前的研究证实,核因子红细胞2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)和血红素加氧酶1(heme oxygenase 1,HO-1)信号通路直接位于活性氧的下游[27],它参与调节抗氧化反应元件依赖基因的转录,以及平衡氧化介质和维持细胞氧化还原稳态,因此也被认为是铁死亡的重要调节因子[28-29]。 研究发现,内源性抗氧化剂褪黑素通过激活Nrf2/HO-1信号通路,降低暴露于高糖的MC3T3-E1细胞中过氧化脂和活性氧的水平,增加GPX4和溶质载体家族7成员11的活性,并抑制高糖诱导的铁死亡。这反过来又增强了成骨能力,促进了骨小梁的形成,增加了骨矿物质密度和相对于总组织体积以及骨小梁数量的骨体积,并改善了骨微结构。siRNA敲除Nrf2抑制了褪黑素对成骨细胞活性的有益作用。研究表明,Nrf2信号通路在褪黑素对骨质疏松的干预中发挥了关键作用[9],还发现全髋关节置换手术失败和翻修最常见的原因之一是无菌性松动。无菌性松动可能是由植入物表面释放的颗粒诱导的骨溶解引起的,导致MC3T3-E1成骨细胞程序性死亡。 氧化亚铜纳米颗粒通过下Nrf2-抗氧化反应元件信号通路和诱导植入物周围骨溶解,促进成骨细胞的铁死亡。因此,用铁素抑制素1和Nrf2激活剂吡噻硫酮阻断铁死亡显著改善了颗粒植入引起的骨溶解,这为治疗无菌性松动提供了一种潜在的策略[30],见图4。线粒体铁蛋白(FtMt)是一种储存铁离子并具有铁氧化酶活性的蛋白质。它可以减少线粒体中游离Fe2+的量,防止过量Fe2+发生芬顿反应,从而降低活性氧的含量[31]。WANG等[26]发现,在高糖条件下,成骨细胞中线粒体二价金属转运蛋白1的表达增加导致铁超载,FtMt的过表达可降低细胞内活性氧水平并抑制成骨细胞的铁死亡。然而,沉默FtMt通过ROS/PINK1/Parkin途径诱导线粒体吞噬,促进了成骨细胞的铁死亡,加速了骨质疏松的病理过程。总之,成骨细胞中铁死亡的抑制减缓了骨质疏松的发展。褪黑激素可以通过激活Nrf2/HO-1途径显著降低铁死亡水平,从而提高MC3T3-E1的成骨能力,见表2。 "
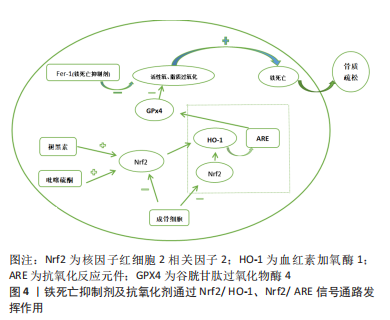
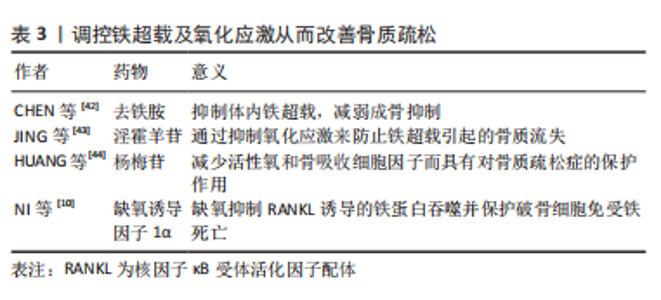
氧化亚铜纳米颗粒下调Nrf2-抗氧化反应元件信号通路可促进成骨细胞的铁死亡,这是影响骨溶解的潜在机制。铁素抑制素1和吡噻硫酮的联合应用显著改善了成骨细胞铁死亡和骨溶解的严重程度,而FtMt缺乏通过ROS/PINK1/Parkin途径诱导线粒体吞噬,从而导致成骨细胞的铁死亡,导致2型糖尿病性骨质疏松。这些结果表明FtMt可能是2型糖尿病性骨质疏松治疗的潜在靶点。 2.2.2 铁死亡和破骨细胞 铁蛋白是一种储存过量细胞铁的蛋白质,当细胞处于缺铁状态时可能会被降解,称为“铁蛋白自噬”,这将增加对细胞内Fe2+引起的铁死亡的敏感性[34]。破骨细胞作为酸分泌细胞和吸收性骨细胞,含有丰富的线粒体,以维持高能量需求[35]。因此,成熟破骨细胞比其他骨细胞需要更多的细胞内游离铁[36]。研究发现,铁蛋白自噬是由铁蛋白重链核受体辅激活因子4复合自噬体降解引发的过程。在有氧条件下,它被核因子κB配体的受体激活剂激活。缺铁反应的发生(转铁蛋白受体1升高,铁蛋白降低)显著增加了丙二醛水平,并诱导破骨细胞铁死亡。相反,缺氧诱导因子1α可以减少RANKL诱导的铁蛋白自噬和缺氧条件下的自噬通量,并保护破骨细胞免于铁死亡。体内研究进一步表明,缺氧诱导因子1α特异性抑制剂2-甲氧基雌二醇可防止去卵巢小鼠的骨丢失[10]。研究还发现,青蒿素及其相关化合物不仅在临床上被用作抗疟药物,而且还被用作治疗骨质流失的潜在替代药物,可能的机制是青蒿素化合物在破骨细胞分化过程中显著增加转铁蛋白受体1介导的铁摄取。 在酸性内体中,Fe3+被还原为Fe2+,Fe2+通过二价金属离子转运体1被释放到细胞质中作为易铁池。此外,青蒿素化合物通过下调RANKL诱导的破骨细胞生成所涉及的途径,选择性地抑制破骨细胞分化,导致破骨细胞铁死亡[37]。总之,青蒿素及其相关化合物在非细胞毒性浓度下显著抑制RANKL诱导的骨髓来源巨噬细胞向破骨细胞的分化,而2-甲氧基雌二醇通过上调RANKL而诱导铁蛋白自噬。两者都会导致破骨细胞的铁死亡,减少破骨细胞生成,并抑制骨吸收,这可能是骨质疏松的替代治疗方法。尽管青蒿素最初是从青蒿中分离出来的,并且在疟疾治疗中有很长的安全应用历史,但仍不清楚青蒿素化合物是否具有不良反应,如低钙血症。需要进一步的研究来评估与目前用于确保青蒿素疗效和安全性的其他抗骨质疏松药物的可能相互作用。 2.2.3 铁死亡和骨细胞 骨细胞活力的丧失是骨质疏松症的一个致病因素。然而,骨细胞死亡的机制尚不清楚,很少有研究以骨细胞为靶细胞来探讨骨质疏松症中铁死亡的机制。最近的一项研究发现,糖尿病微环境显著增强了体外骨细胞脱铁,如大量脂质过氧化和铁超载。根据RNA测序结果,骨细胞中HO-1表达显著上调。HO-1对于糖尿病性骨质疏松中的骨细胞铁死亡至关重要[38]。靶向铁死亡(注射铁死亡抑制剂Fer-1)或HO-1(注射锌原卟啉,HO-1抑制剂)可以通过破坏脂质过氧化来逆转糖尿病性骨质疏松中的骨细胞死亡,最终逆转骨丢失。 铁超载诱导MLO-Y4骨细胞样细胞和原代骨细胞凋亡。过量的铁显著促进MLO-Y4细胞中RANKL的表达,促进破骨细胞分化并增加骨吸收能力[39]。因此,不可否认的是,骨细胞中铁死亡的调控也成为治疗骨质疏松症的新靶点。 2.3 骨质疏松症的新治疗靶点——铁死亡 铁超载和活性氧积累是铁死亡的主要特征,脂质过氧化是其关键,因此,调节铁死亡需要它们作为靶点。例如,氧化应激诱导的铁死亡涉及椎间盘退变的进展,椎间盘退变是骨代谢疾病之一。铁超载在过氧叔丁醇诱导的人类髓核细胞铁死亡中起着重要作用[40]。众所周知,扁豆醇是一种生物活性化合物,可以抵抗活性氧并特异性恢复铁泵蛋白功能,它的使用可以逆转椎间盘退变的进展,这表明抑制铁凋亡可以减缓椎间盘退变的进程[41]。作为另一种骨代谢疾病,骨质疏松与活性氧的积累有关。类似地,铁过载也参与骨质疏松的形成,铁超载和活性氧积累是铁死亡的显著特征。因此,调节铁死亡可以成为骨质疏松症治疗的新目标。见表3。"
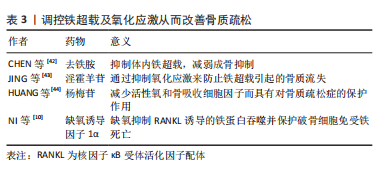
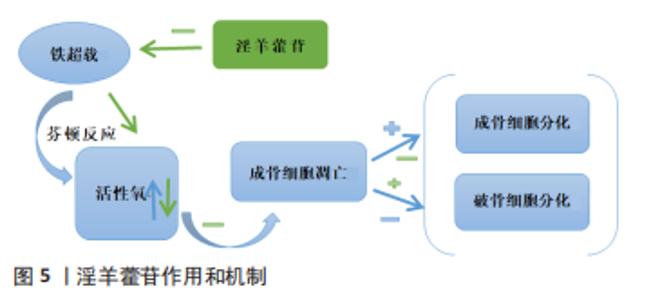
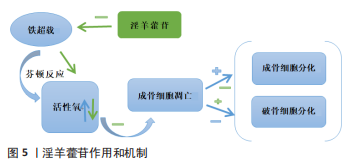
2.3.1 铁过载调节 铁螯合剂对铁超载引起的骨质疏松症有极好的治疗效果。目前临床上使用的3种铁螯合剂是去铁胺、去铁酮和去铁罗[45]。去铁胺在骨质疏松症中的作用机制主要表现如下:首先,在缺乏pVHL的小鼠中,成骨细胞中的缺氧诱导因子1α激活可以显著增加血管生成,并产生更多的骨,以响应牵张成骨[46]。去铁胺是一种缺氧诱导因子1α激活剂,可以增加血管生成和成骨。成骨细胞中缺氧诱导因子通路的条件激活可以增加血管生成和成骨,并保护小鼠免受去卵巢诱导的骨丢失[47]。 其次,去铁胺通过Wnt/β-catenin信号通路刺激间充质干细胞的骨形成[48],并通过Nrf2相关因子介导的抗氧化信号通路促进人牙周膜细胞的成骨细胞分化[49]。最后,去铁胺逆转了骨质疏松模型中升高的铁水平并降低了铁负荷[50]。斑马鱼体内的铁超载显著抑制骨形成,同时伴有成骨细胞特异性基因的表达减少[42]。铁对成骨细胞和骨形成的不良影响可能与活性氧诱导的氧化应激增加有关,去铁胺能减少铁过载幼年斑马鱼的氧化应激,从而促进幼年斑马鱼骨形成。去铁胺可以逆转辐射诱导的铁水平升高和骨小梁退化,并在体外辐射环境中抑制破骨细胞分化[51]。 铁蛋白是一种储存铁的蛋白质,当细胞缺乏铁时,它会降解产生游离铁。游离铁通过与核受体共激活因子4结合传递到早期自噬囊泡,然后导致其降解并释放到细胞质中[52]。此外,线粒体中的铁-硫簇在富氧环境中快速降解[53],铁-硫簇的丢失可导致转铁蛋白受体1增加和铁蛋白重链减少,促进铁死亡。破骨细胞是具有丰富线粒体的高能量需求细胞[54-55]。成熟的破骨细胞需要比其他细胞更多的细胞内游离铁来满足线粒体的生物发生和能量需求。因此,一旦线粒体铁-硫簇降解,破骨细胞可能容易发生铁死亡。缺氧可抑制铁蛋白吞噬,保护破骨细胞免于铁死亡。缺氧诱导因子1α抑制剂二甲氧基雌二醇通过促进破骨细胞铁死亡来预防去卵巢诱导的小鼠骨质疏松症。外泌体是细胞间通信的重要媒介,参与许多生理和病理过程[56]。内皮细胞的外泌体可以通过抑制铁蛋白依赖性铁死亡,逆转糖皮质激素引起的成骨抑制[57-58]。 过量的铁和脂质过氧化在铁死亡中是不可或缺的。游离不稳定的铁和脂质过氧化物通过芬顿反应产生大量活性氧,从而导致铁死亡。因此,可以通过调节铁过载以及解决氧化应激来控制铁死亡,两者是相互关联和互补的。 2.3.2 氧化应激的调节 活性氧的大量积累是铁死亡的重要特征之一,氧化应激也是骨质疏松的重要致病因素。过量的铁会破坏骨组织的氧化还原平衡,导致抗氧化剂的减少和脂质过氧化物的积累,从而影响DNA复制并导致细胞死亡。淫羊藿苷具有抗氧化和抗骨质疏松作用。铁超载可通过芬顿反应产生大量活性氧,诱导成骨细胞凋亡,抑制成骨细胞分化,促进破骨细胞分化。口服淫羊藿苷通过逆转这些效应,显著防止铁超载小鼠的骨质流失[43],见图5。"

不难看出,抗氧化剂可以通过调节铁代谢来维持正常的氧化还原稳态,尤其是在铁过载的情况下。杨梅苷是一种从植物中分离出来的强效抗氧化剂。在氧化应激诱导的细胞损伤模型中加入杨梅苷可降低RANKL表达和活性氧产生,降低血清丙二醛水平,并增加谷胱甘肽活性。在动物模型中,它的使用改善了骨骼的微观结构。由此可见杨梅苷对骨质疏松症的治疗作用与调节氧化应激介导的铁死亡有关[44]。维生素C可以预防骨质疏松[59],它增加了去卵巢大鼠的成骨基因表达和抗氧化能力[60-61]。抗氧化剂的使用在骨质疏松症的治疗中起着至关重要的作用。例如,茶多酚的骨保护作用是通过抗氧化或抗炎机制介导的[62]。假蛋白A可以抑制破骨细胞的生成和活性氧水平的升高,防止去卵巢诱导的骨丢失[63]。最近的一项研究表明,一种新的以RANKL为靶点的黄酮苷通过抑制活化T细胞核因子1和活性氧来预防骨质疏松症[64]。这些结果表明,抗氧化剂可以防止更多的活性氧产生,抑制骨吸收,从而改善骨代谢,有效预防骨质疏松症的发生。 高糖诱导成骨细胞毒性和脂质过氧化物积累,通过增加活性氧、脂质过氧化和谷胱甘肽耗竭而导致铁死亡。然而,这些效应被铁死亡抑制剂和铁螯合剂逆转[9]。Nrf2是活性氧的直接下游,是调节细胞抗氧化反应的关键转录因子,Nrf2在减轻脂质过氧化和调节铁死亡中发挥关键作用 [28]。在Nrf2激活的许多抗氧化酶中,HO-1具有更好的抗氧化能力并抑制细胞凋亡,HO-1对于糖尿病微环境诱导的铁死亡至关重要[38]。通过激活Nrf2/HO-1途径,褪黑素可以显著减少MC3T3-E1细胞中的铁死亡,并提高成骨能力[9]。 GPX4和硒蛋白是一种重要的抗氧化酶,可抑制铁死亡。GPX4通过将反应性醛还原为醇形式起到抗氧化剂的作用[65]。硒通过形成第21个氨基酸硒半胱氨酸参与GPX4合成的分子机制已经阐明[66]。与铁一样,硒在铁死亡中也起着决定性作用[67-69]。依布硒是一种具有抗炎和抗氧化特性的无毒有机硒药物,通过调节RANKL/骨保护素比值抑制破骨细胞的形成,恢复小鼠模型中脂多糖介导的骨丢失[70]。"

| [1] LANE NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194(2 Suppl):S3-11. [2] SRIVASTAVA M, DEAL C. Osteoporosis in elderly: prevention and treatment. Clin Geriatr Med. 2002;18(3):529-555. [3] BACCARO LF, CONDE DM, COSTA-PAIVA L, et al. The epidemiology and management of postmenopausal osteoporosis: a viewpoint from Brazil. Clin Interv Aging. 2015;10:583-591. [4] WEINSTEIN RS, MANOLAGAS SC. Apoptosis and osteoporosis. Am J Med. 2000;108(2):153-164. [5] KIM KH, LEE MS. Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10(6):322-337. [6] TIAN Q, WU S, DAI Z, et al. Iron overload induced death of osteoblasts in vitro: involvement of the mitochondrial apoptotic pathway. PeerJ. 2016;4: e2611. [7] TIAN Q, QIN B, GU Y, et al. ROS-Mediated Necroptosis Is Involved in Iron Overload-Induced Osteoblastic Cell Death. Oxid Med Cell Longev. 2020; 2020:1295382. [8] GE W, JIE J, YAO J, et al. Advanced glycation end products promote osteoporosis by inducing ferroptosis in osteoblasts. Mol Med Rep. 2022; 25(4):140. [9] MA H, WANG X, ZHANG W, et al. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxid Med Cell Longev. 2020;2020:9067610. [10] NI S, YUAN Y, QIAN Z, et al. Hypoxia inhibits RANKL-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radic Biol Med. 2021;169: 271-282. [11] DING H, CHEN S, PAN X, et al. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J Cachexia Sarcopenia Muscle. 2021;12(3):746-768. [12] LIU P, WANG W, LI Z, et al. Ferroptosis: A New Regulatory Mechanism in Osteoporosis. Oxid Med Cell Longev. 2022;2022:2634431. [13] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072. [14] DIXON SJ, STOCKWELL BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9-17. [15] STOCKWELL BR, FRIEDMANN ANGELI JP, BAYIR H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273-285. [16] STRASSER A, O’CONNOR L, DIXIT VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217-245. [17] WANG X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922-2933. [18] KROEMER G, GALLUZZI L, VANDENABEELE P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3-11. [19] VANLANGENAKKER N, VANDEN BERGHE T, VANDENABEELE P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19(1):75-86. [20] BERGMANN A. Autophagy and cell death: no longer at odds. Cell. 2007; 131(6):1032-1034. [21] YANG Y, KLIONSKY DJ. Autophagy and disease: unanswered questions. Cell Death Differ. 2020;27(3):858-871. [22] LEDESMA-COLUNGA MG, WEIDNER H, VUJIC SPASIC M, et al. Shaping the bone through iron and iron-related proteins. Semin Hematol. 2021; 58(3):188-200. [23] LIU F, ZHANG WL, MENG HZ, et al. Regulation of DMT1 on autophagy and apoptosis in osteoblast. Int J Med Sci. 2017;14(3):275-283. [24] MENG HZ, ZHANG WL, LIU F, et al. Advanced Glycation End Products Affect Osteoblast Proliferation and Function by Modulating Autophagy Via the Receptor of Advanced Glycation End Products/Raf Protein/Mitogen-activated Protein Kinase/Extracellular Signal-regulated Kinase Kinase/Extracellular Signal-regulated Kinase (RAGE/Raf/MEK/ERK) Pathway. J Biol Chem. 2015;290(47):28189-28199. [25] ZHANG WL, MENG HZ, YANG MW. Regulation of DMT1 on Bone Microstructure in Type 2 Diabetes. Int J Med Sci. 2015;12(5):441-449. [26] WANG X, MA H, SUN J, et al. Mitochondrial Ferritin Deficiency Promotes Osteoblastic Ferroptosis Via Mitophagy in Type 2 Diabetic Osteoporosis. Biol Trace Elem Res. 2022;200(1):298-307. [27] ZHANG DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38(4):769-789. [28] DODSON M, CASTRO-PORTUGUEZ R, ZHANG DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. [29] SONG X, LONG D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front Neurosci. 2020;14:267. [30] XU Y, SANG W, ZHONG Y, et al. CoCrMo-Nanoparticles induced peri-implant osteolysis by promoting osteoblast ferroptosis via regulating Nrf2-ARE signalling pathway. Cell Prolif. 2021;54(12):e13142. [31] LEVI S, CORSI B, BOSISIO M, et al. A human mitochondrial ferritin encoded by an intronless gene. J Biol Chem. 2001;276(27):24437-24440. [32] KIM EN, TRANG NM, KANG H, et al. Phytol Suppresses Osteoclast Differentiation and Oxidative Stress through Nrf2/HO-1 Regulation in RANKL-Induced RAW264.7 Cells. Cells. 2022;11(22):3596. [33] XIAO J, ZHANG G, CHEN B, et al. Quercetin protects against iron overload-induced osteoporosis through activating the Nrf2/HO-1 pathway. Life Sci. 2023;322:121326. [34] QIN Y, QIAO Y, WANG D, et al. Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications. Biomed Pharmacother. 2021;141:111872. [35] ARNETT TR, ORRISS IR. Metabolic properties of the osteoclast. Bone. 2018; 115:25-30. [36] ISHII KA, FUMOTO T, IWAI K, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15(3):259-266. [37] ZHANG J. The osteoprotective effects of artemisinin compounds and the possible mechanisms associated with intracellular iron: A review of in vivo and in vitro studies. Environ Toxicol Pharmacol. 2020;76:103358. [38] YANG Y, LIN Y, WANG M, et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022;10(1):26. [39] YANG J, DONG D, LUO X, et al. Iron Overload-Induced Osteocyte Apoptosis Stimulates Osteoclast Differentiation Through Increasing Osteocytic RANKL Production In Vitro. Calcif Tissue Int. 2020;107(5):499-509. [40] YANG RZ, XU WN, ZHENG HL, et al. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J Cell Physiol. 2021;236(4):2725-2739. [41] LU S, SONG Y, LUO R, et al. Ferroportin-Dependent Iron Homeostasis Protects against Oxidative Stress-Induced Nucleus Pulposus Cell Ferroptosis and Ameliorates Intervertebral Disc Degeneration In Vivo. Oxid Med Cell Longev. 2021;2021:6670497. [42] CHEN B, YAN YL, LIU C, et al. Therapeutic effect of deferoxamine on iron overload-induced inhibition of osteogenesis in a zebrafish model. Calcif Tissue Int. 2014;94(3):353-360. [43] JING X, DU T, CHEN K, et al. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J Cell Physiol. 2019;234(7):10123-10137. [44] HUANG Q, GAO B, WANG L, et al. Protective effects of myricitrin against osteoporosis via reducing reactive oxygen species and bone-resorbing cytokines. Toxicol Appl Pharmacol. 2014;280(3):550-560. [45] WU D, WEN X, LIU W, et al. Comparison of the effects of deferasirox, deferoxamine, and combination of deferasirox and deferoxamine on an aplastic anemia mouse model complicated with iron overload. Drug Des Devel Ther. 2018;12:1081-1091. [46] WAN C, GILBERT SR, WANG Y, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008;105(2):686-691. [47] ZHAO Q, SHEN X, ZHANG W, et al. Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone. 2012;50(3):763-770. [48] CHE J, YANG J, ZHAO B, et al. The Effect of Abnormal Iron Metabolism on Osteoporosis. Biol Trace Elem Res. 2020;195(2):353-365. [49] CHUNG JH, KIM YS, NOH K, et al. Deferoxamine promotes osteoblastic differentiation in human periodontal ligament cells via the nuclear factor erythroid 2-related factor-mediated antioxidant signaling pathway. J Periodontal Res. 2014;49(5):563-573. [50] XU Z, SUN W, LI Y, et al. The regulation of iron metabolism by hepcidin contributes to unloading-induced bone loss. Bone. 2017;94:152-161. [51] ZHANG J, ZHENG L, WANG Z, et al. Lowering iron level protects against bone loss in focally irradiated and contralateral femurs through distinct mechanisms. Bone. 2019;120:50-60. [52] DOWDLE WE, NYFELER B, NAGEL J, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16(11):1069-1079. [53] ALVAREZ SW, SVIDERSKIY VO, TERZI EM, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017; 551(7682):639-643. [54] LEMMA S, SBOARINA M, PORPORATO PE, et al. Energy metabolism in osteoclast formation and activity. Int J Biochem Cell Biol. 2016;79:168-180. [55] LI B, LEE WC, SONG C, et al. Both aerobic glycolysis and mitochondrial respiration are required for osteoclast differentiation. FASEB J. 2020;34(8): 11058-11067. [56] WORTZEL I, DROR S, KENIFIC CM, et al. Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell. 2019;49(3):347-360. [57] LU J, YANG J, ZHENG Y, et al. Extracellular vesicles from endothelial progenitor cells prevent steroid-induced osteoporosis by suppressing the ferroptotic pathway in mouse osteoblasts based on bioinformatics evidence. Sci Rep. 2019;9(1):16130. [58] YANG RZ, XU WN, ZHENG HL, et al. Exosomes derived from vascular endothelial cells antagonize glucocorticoid-induced osteoporosis by inhibiting ferritinophagy with resultant limited ferroptosis of osteoblasts. J Cell Physiol. 2021;236(9):6691-6705. [59] FINCK H, HART AR, JENNINGS A, et al. Is there a role for vitamin C in preventing osteoporosis and fractures? A review of the potential underlying mechanisms and current epidemiological evidence. Nutr Res Rev. 2014; 27(2):268-283. [60] DEYHIM F, STRONG K, DEYHIM N, et al. Vitamin C reverses bone loss in an osteopenic rat model of osteoporosis. Int J Vitam Nutr Res. 2018;88(1-2): 58-64. [61] SZARKA A, KAPUY O, LŐRINCZ T, et al. Vitamin C and Cell Death. Antioxid Redox Signal. 2021;34(11):831-844. [62] SHEN CL, CHYU MC, WANG JS. Tea and bone health: steps forward in translational nutrition. Am J Clin Nutr. 2013;98(6 Suppl):1694S-1699S. [63] CHEN K, QIU P, YUAN Y, et al. Pseurotin A Inhibits Osteoclastogenesis and Prevents Ovariectomized-Induced Bone Loss by Suppressing Reactive Oxygen Species. Theranostics. 2019;9(6):1634-1650. [64] HONG G, CHEN Z, HAN X, et al. A novel RANKL-targeted flavonoid glycoside prevents osteoporosis through inhibiting NFATc1 and reactive oxygen species. Clin Transl Med. 2021;11(5):e392. [65] BERSUKER K, HENDRICKS JM, LI Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688-692. [66] INGOLD I, BERNDT C, SCHMITT S, et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018;172(3): 409-422.e21. [67] CONRAD M, PRONETH B. Selenium: Tracing Another Essential Element of Ferroptotic Cell Death. Cell Chem Biol. 2020;27(4):409-419. [68] ALIM I, CAULFIELD JT, CHEN Y, et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell. 2019;177(5): 1262-1279.e25. [69] DOUGALL WC. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res. 2012;18(2):326-335. [70] BAEK JM, KIM JY, YOON KH, et al. Ebselen Is a Potential Anti-Osteoporosis Agent by Suppressing Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Osteoclast Differentiation In vitro and Lipopolysaccharide-Induced Inflammatory Bone Destruction In vivo. Int J Biol Sci. 2016;12(5):478-488. |
| [1] | Guo Sutong, Feng Dehong, Guo Yu, Wang Ling, Ding Yujian, Liu Yi, Qian Zhengying, Li Mingyang. Construction and finite element analysis of normal and osteoporotic hip models [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1342-1346. |
| [2] | Yu Weijie, Liu Aifeng, Chen Jixin, Guo Tianci, Jia Yizhen, Feng Huichuan, Yang Jialin. Advantages and application strategies of machine learning in diagnosis and treatment of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1426-1435. |
| [3] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [4] | Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300. |
| [5] | Huang Haoran, Fan Yinuo, Wei-Yang Wenxiang, Jiang Mengyu, Fang Hanjun, Wang Haibin, Chen Zhenqiu, Liu Yuhao, Zhou Chi. Urolithin A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1149-1154. |
| [6] | Zhang Xiaoyun, Liu Hua, Chai Yuan, Chen Feng, Zeng Hao, Gao Zhengang, Huang Yourong. Effect of Yishen Gushu Formula on bone metabolic markers and clinical efficacyn in patients with osteoporosis of kidney deficiency and blood stasis type [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1155-1160. |
| [7] | Shen Jiangyong, He Xi, Tang Yuting, Wang Jianjun, Liu Jinyi, Chen Yuanyuan, Wang Xinyi, Liu Tong, Sun Haoyuan. RAS-selective lethal small molecule 3 inhibits the fibrosis of pathological scar fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1168-1173. |
| [8] | Dai Yuexing, Zheng Liqin, Wu Minhui, Li Zhihong, Li Shaobin, Zheng Desheng, Lin Ziling. Effect of vessel number on computational fluid dynamics in vascular networks [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1206-1210. |
| [9] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [10] | Wang Wen, Zheng Pengpeng, Meng Haohao, Liu Hao, Yuan Changyong. Overexpression of Sema3A promotes osteogenic differentiation of dental pulp stem cells and MC3T3-E1 [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 993-999. |
| [11] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [12] | Zhang Min, Peng Jing, Zhang Qiang, Chen Dewang. Mechanical properties of L3/4 laminar decompression and intervertebral fusion in elderly osteoporosis patients analyzed by finite element method [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 847-851. |
| [13] | Xue Xiaofeng, Wei Yongkang, Qiao Xiaohong, Du Yuyong, Niu Jianjun, Ren Lixin, Yang Huifeng, Zhang Zhimin, Guo Yuan, Chen Weiyi. Finite element analysis of osteoporosis in proximal femur after cannulated screw fixation for femoral neck fracture [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 862-867. |
| [14] | Kaiyisaier•Abudukelimu, Maimaitimin•Abulimiti, Li Lei, Yang Xiaokai, Zhang Yukun, Liu Shuai. Effect of lumbar CT values in the diagnosis of osteoporosis in women patients with lumbar degenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 945-949. |
| [15] | Wang Liping, Lian Tianxing, Hu Yongrong, Yang Hongsheng, Zeng Zhimou, Liu Hao, Qu Bo. HU value of chest CT vertebral body in the opportunistic screening of type 2 diabetes mellitus osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 950-954. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||