Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (1): 131-138.doi: 10.12307/2023.742
Previous Articles Next Articles
Astrocytes regulate glial scar formation in cerebral ischemic stroke
Yang Ting1, Ding Zhibin1, 2, 3, Jiang Nan1, Han Hongxia4, Hou Miaomiao1, 3, Ma Cungen2, Song Lijuan1, 2, 3, Li Xinyi1, 3
- 1Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China; 2The Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Department of Encephalopathy, First Clinical College, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China; 3Key Laboratory of Cellular Physiology, Shanxi Medical University, Ministry of Education, Taiyuan 030001, Shanxi Province, China; 4Shanxi Cardiovascular Hospital (Affiliated Cardiovascular Hospital of Shanxi Medical University), Taiyuan 030024, Shanxi Province, China
-
Received:2022-11-02Accepted:2022-12-10Online:2024-01-08Published:2023-06-29 -
Contact:Li Xinyi, Chief physician, Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China; Key Laboratory of Cellular Physiology, Shanxi Medical University, Ministry of Education, Taiyuan 030001, Shanxi Province, China Song Lijuan, MD, Associate professor, Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China; The Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Department of Encephalopathy, First Clinical College, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China; Key Laboratory of Cellular Physiology, Shanxi Medical University, Ministry of Education, Taiyuan 030001, Shanxi Province, China -
About author:Yang Ting, Master candidate, Physician, Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China Ding Zhibin, MD, Attending physician, Department of Neurology, Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital), Taiyuan 030032, Shanxi Province, China; The Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Department of Encephalopathy, First Clinical College, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China; Key Laboratory of Cellular Physiology, Shanxi Medical University, Ministry of Education, Taiyuan 030001, Shanxi Province, China -
Supported by:Youth Science Foundation Program of National Natural Science Foundation of China, No. 82004028 (to SLJ); General Program Supported by China Postdoctoral Science Foundation, No. 2020M680912 (to SLJ); Free Exploration Project of Shanxi Basic Research Priorities Program, No. 20210302123408 (to DZB); Leading Medical Science and Technology Team of Shanxi Health Commission, No. 2020TD05 (to MCG); Talent Introduction Program of Scientific Research Foundation Supported by Third Hospital of Shanxi Medical University, No. 2021RC033 (to DZB); Young Scientist Development Program Supported by Shanxi University of Chinese Medicine, No. 2021-PY-QN-09 (to SLJ)
CLC Number:
Cite this article
Yang Ting, Ding Zhibin, Jiang Nan, Han Hongxia, Hou Miaomiao, Ma Cungen, Song Lijuan, Li Xinyi. Astrocytes regulate glial scar formation in cerebral ischemic stroke[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 131-138.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
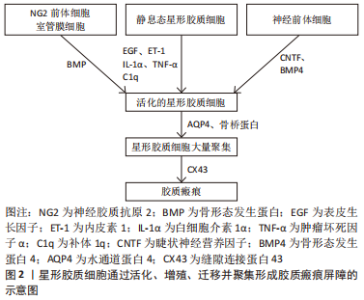
2.1 星形胶质细胞形成缺血性脑卒中胶质瘢痕的过程 2.1.1 星形胶质细胞的活化过程 星形胶质细胞作为中枢神经系统中数量最多、分布最广泛的细胞,在支撑大脑结构和维持大脑生理功能方面有着重要作用。在结构上,星形胶质细胞主要参与血脑屏障的形成,从而为维持中枢神经系统生理功能提供稳定的内环境[13]。在功能上,星形胶质细胞的作用可分为3个层面:在分子层面上,星形胶质细胞维持离子、水和神经递质稳态,参与代谢支持和活性氧去除;在细胞层面上,星形胶质细胞不仅能与小胶质细胞和少突胶质细胞协同参与炎症过程,还可促进神经发生和突触形成、成熟及重塑;在系统层面上,星形胶质细胞调节脑血流和脑淋巴液回流,是清除脑代谢产物的结构基础[14-15]。 缺血性脑卒中诱导受损神经元和小胶质细胞释放炎性因子,促使星形胶质细胞中数千个基因上调,特征性的胶质纤维酸性蛋白(Glial fibrillary acidic protein,GFAP)水平升高,星形胶质细胞从静息态转变为激活态,表现为增生和肥大,在受损部位形成胶质界限,这一过程称为反应性星形胶质细胞增生。反应性星形胶质细胞在缺血性脑卒中不同受损部位和损伤时间的差异性反应提示其功能的高度异质性。二代基因测序从分子水平再次证实了星形胶质细胞的区域异质性及其对损伤的差异性反应[16]。反应性星形胶质细胞可分为A1和A2两种亚型:A1型反应性星形胶质细胞由小胶质细胞分泌的白细胞介素1α、肿瘤坏死因子α和补体1q (Complement 1q,C1q)诱导活化后,可促进脑缺血区域的神经炎症反应,进而导致神经元损伤和突触结构破坏;A2型反应性星形胶质细胞在缺血条件下被诱导,通过分泌神经营养因子促进神经再生和突触修复[17-18]。根据脑缺血损伤的严重程度或相对损伤的距离不同,反应性星形胶质细胞增生呈现从肿胀、增殖到胶质瘢痕形成的动态变化[19]。 2.1.2 星形胶质细胞形成胶质瘢痕屏障 在缺血性脑卒中后超早期,小胶质细胞表达骨桥蛋白诱导星形胶质细胞迁移,并通过整合素受体αvβ3将星形胶质细胞定位于梗死区周围,成为胶质瘢痕的主要成分[20]。缺血性脑卒中诱导表皮生长因子、内皮素1(Endothelin-1,ET-1)和炎性递质上调,触发成熟的星形胶质细胞重新进入细胞周期并增殖,从而成为瘢痕样星形胶质细胞的最主要来源方式[21-22]。其次,从远端区域迁移到病变部位的星形胶质细胞是瘢痕样星形胶质细胞的另一个重要来源方式。 水通道蛋白4(Aquaporin 4,AQP4)具有黏附性,能迅速改变星形胶质细胞足突的体积进而促进其迁移,并且星形胶质细胞表面AQP4的重组和向瘢痕中心的再分布是早期瘢痕形成的关键步骤[23-24]。此外,脑卒中后睫状神经营养因子(Ciliary neurotrophic factor,CNTF)、骨形态发生蛋白4(Bone morphogenetic protein 4,BMP4)的上调诱导了神经干细胞向反应性星形胶质细胞的分化;脑实质局部的神经胶质抗原2(Neuron-glial antigen 2,NG2)前体细胞和室管膜细胞前体细胞也可分化为星形胶质细胞,从而参与瘢痕形成[15,25-27]。反应性星形胶质细胞还通过上调细胞表面连接蛋白形成缝隙连接,从而通过增强细胞间联系进一步加强胶质瘢痕屏障[15]。总之,多种来源的星形胶质细胞经过激活、增殖和迁移共同参与了胶质瘢痕的形成,见图2,这些细胞表型的变化受许多不同的信号分子调节。聚集在缺血病灶周围的反应性星形胶质细胞表现为细胞突起拉长、交错、结合形成重叠束,通过重新定向并组织成致密的网状瘢痕结构,成为阻碍轴突再生的物理屏障。"
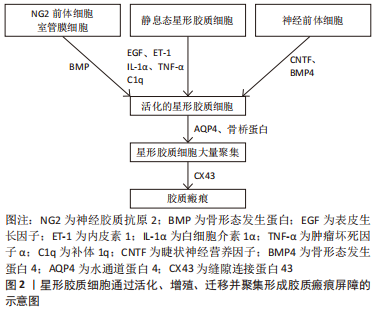
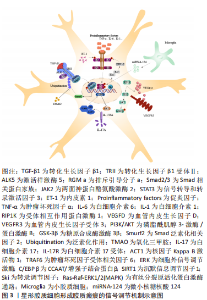
2.1.3 星形胶质细胞产生细胞外基质增强胶质瘢痕屏障 反应性星形胶质细胞不仅通过聚集形成胶质瘢痕,还可向梗死周围区域分泌细胞外基质蛋白,从而丰富胶质瘢痕的抑制环境。反应性星形胶质细胞最早合成硫酸软骨素蛋白多糖(Chondroitin sulfate proteoglycan,CSPG),形成了细胞外基质的主要成分,且CSPG的浓度随梗死时间的推移而逐渐增高。CSPG既与酪氨酸磷酸酶受体家族相互作用,使轴突远端发生营养不良和塌陷,还与神经轴突生长抑制因子(Neurite outgrowth inhibitor,Nogo)受体(Nogo受体1和Nogo受体3)结合阻止神经干细胞向损伤区域迁移,影响缺血性脑卒中后的神经再生[28]。星形胶质细胞还可分泌纤维连接蛋白、层粘连蛋白和张力蛋白,这些细胞外基质蛋白沉积在胶质瘢痕内,与星形胶质细胞共同形成一道严密的物理分子屏障。脑卒中后生长因子水平的增加促使神经修复自发进行,其中受损的轴突从切断的轴突残端形成生长锥,引导轴突进行极化和延伸。然而,胶质瘢痕限制了轴突穿过受损部位的潜能,表现为再生轴突从病变部位回缩或者呈现碎片化的退行性变化,或者形成肿胀和无序末端[29-30]。胶质瘢痕虽在炎症高峰期对组织损伤的限制必不可少,但在缺血性脑卒中恢复期却严重阻碍了轴突生长,成为影响神经修复过程的主要障碍。此外,胶质瘢痕的持续存在还会引起认知功能损害。在缺血性脑卒中慢性期,缺血脑组织发生液化坏死,胶质瘢痕可诱导多种神经毒性因子趋化,致使液化坏死区附近的神经元处于持续的慢性炎症反应中,这可能是梗死后患者出现迟发性脑萎缩和痴呆的重要原因[31]。 总之,星形胶质细胞在缺血性脑卒中的病理过程中扮演重要的角色。根据脑组织缺血损伤的程度,星形胶质细胞的活化呈现不同的反应性。严重的脑组织损伤往往刺激星形胶质细胞过度活化,表现为大量增殖、迁移并聚集在损伤周围,是形成胶质瘢痕的主要成分。此外,星形胶质细胞还通过分泌以CSPG为代表的细胞外基质,进一步增强了胶质瘢痕的稳定性。胶质瘢痕是脑卒中慢性期神经修复的主要障碍,阻止了神经前体细胞的迁移和轴突的延伸,因此,抑制胶质瘢痕的形成对神经功能的恢复具有积极作用。星形胶质细胞在缺血性脑卒中后形成胶质瘢痕的过程是一个多细胞、多分子参与的复杂过程,深入了解其机制有助于实施有效的措施干预胶质瘢痕在神经修复中的抑制作用。 2.2 星形胶质细胞形成缺血性脑卒中胶质瘢痕的信号调节通路 缺血性脑卒中发生后,多种细胞内外信号分子的表达发生改变,通过调控星形胶质细胞活化、增殖和迁移及炎症反应共同调节胶质瘢痕的形成,进而为缺血性脑卒中的治疗提供了更多的干预靶点,见图3。"
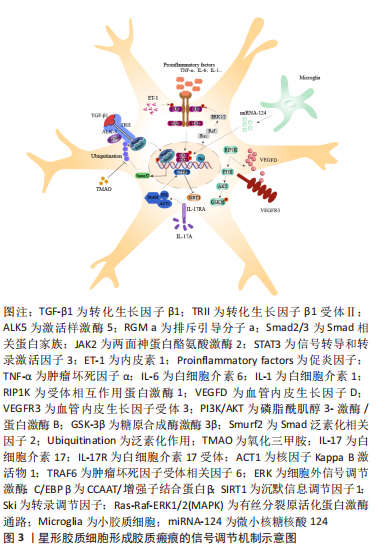

2.2.1 转化生长因子β/Smad信号通路 在中枢神经系统生理和病理条件下,转化生长因子β1和骨形态发生蛋白可上调Smad相关蛋白家族中Smad2和Smad3的表达,进而调节星形胶质细胞的分化。有趣的是,缺血性脑卒中后缺血半暗带区转化生长因子β1表达显著升高,通过激活样激酶5(Activing-like kinase 5,ALK5)与Smad相互作用,诱导星形胶质细胞活化和CSPG沉积,是胶质瘢痕中细胞外基质产生的主要信号通路。转化生长因子β1通过受体ALK5刺激星形胶质细胞表达排斥引导分子a(Repulsive guidance molecule a,RGMa),是胶质瘢痕形成的关键步骤。RGMa与Smad2,Smad3和ALK5形成复合体,促进转化生长因子β1/Smad2/3信号转导,诱导反应性星形胶质细胞活化和胶质瘢痕形成,对神经功能的恢复产生不利影响[32]。转化生长因子β1还是诱导星形胶质细胞迁移的主要因子,以浓度依赖的方式增加神经蛋白聚糖和磷酸化Smad3的表达,促进体外星形胶质细胞迁移。同时,转化生长因子β1还可增加与细胞迁移密切相关的基质金属蛋白酶2(Matrix metalloproteinases 2,MMP-2)、MMP-9的产生。此外,缺血性脑卒中后血脑屏障的破坏导致纤维蛋白原进入中枢神经系统并以纤维蛋白的形式沉积,进而与转化生长因子β结合,诱导星形胶质细胞中的Smad2磷酸化和CSPG沉积,这表明转化生长因子β是血管通透性和胶质瘢痕之间的重要联系分子[33-34]。因此,抑制转化生长因子β/Smad信号通路的关键分子不仅可以调节星形胶质细胞的反应性,还能减少细胞外基质的沉积,理论上是抑制胶质瘢痕形成的有效手段。 2.2.2 JAK/STAT3信号通路 两面神蛋白酪氨酸激酶(Janus kinase,JAK)是细胞内上游信号通路中重要的信使分子,在特定膜受体的刺激下使信号转导和转录激活因子(Signal transducer and activator of transcription,STAT)蛋白家族磷酸化。STAT3在脑内广泛表达,是反应性星形胶质细胞增生症的中心调节因子。急性缺血性脑卒中后,JAK/STAT3通路被激活,在神经元凋亡、神经炎症和星形胶质细胞增生中发挥重要作用。研究发现,敲除STAT3基因的动物模型出现反应性星形胶质细胞增殖和迁移减少,同时梗死周围胶质瘢痕的厚度变薄[8]。 CHENG等[22]研究发现,星形胶质细胞来源的ET-1在脑缺血后表达上调,进一步通过JAK/STAT3通路促进神经前体细胞向星形胶质细胞的分化,参与胶质瘢痕的形成,导致原有脑损伤加重。此外,STAT3的激活可限制激活蛋白24抗体(Ras homolog gene family A,Rho A)的活性,从而控制肌球蛋白张力,促进反应性星形胶质细胞迁移和胶质瘢痕形成[35]。上述研究表明,STAT3在缺血性脑卒中后表达上调,诱导星形胶质细胞反应性增加,从而促进胶质瘢痕形成。而且,白细胞介素1β(Interleukin-1β,IL-1β)在缺血性脑卒中后刺激星形胶质细胞中JAK/STAT3信号转导增加,并诱导星形胶质细胞发生表型转换,发挥神经毒性作用[36]。因此 JAK/STAT3信号的激活可能是细胞因子介导的星形胶质细胞功能调节的基础。 2.2.3 RIP1K/PI3K/AKT/GSK-3β信号通路 缺血性脑卒中后大量炎性递质表达增加,包括白细胞介素1β、白细胞介素6和肿瘤坏死因子α,可诱导反应性星形胶质细胞增生,是胶质瘢痕形成的必要条件。糖原合成酶激酶3β(Glycogen synthase kinase 3β,GSK-3β)和受体相互作用蛋白激酶1(Receptor-interacting protein 1 kinase,RIP1K)作为炎症反应的关键调节因子,可有效抑制炎性递质释放,进而阻碍胶质瘢痕形成[37]。缺血性脑卒中后梗死周围区域的RIP1K含量显著上升,且RIP1K含量变化与星形胶质细胞增生和胶质瘢痕形成呈正相关。RIP1K主要通过增强血管内皮生长因子D(Vascular endothelial growth factor D,VEGFD)/血管内皮生长因子受体3(Vascular endothelial growth factor receptor 3,VEGFR3)信号通路,促进星形胶质细胞胶质瘢痕形成[38]。此外,RIP1K激活的磷脂酰肌醇3-激酶/蛋白激酶B(Phosphatidylinositol 3-kinase/protein kinase B,PI3K/AKT)通路和脑缺血后反应性星形胶质细胞诱导蛋白激酶B(Protein kinase B,AKT)发生磷酸化,均可促使下游GSK-3β活化,在脑缺血过程中的炎症因子释放和胶质瘢痕形成中发挥重要作用[37]。在脑卒中后14 d通过抑制RIP1K和GSK-3β仍能降低胶质瘢痕厚度[11],表明RIP1K和GSK-3β可能是药物治疗的重要治疗靶点。 2.2.4 Smurf2/ALK5信号通路 Smad泛素化相关因子2(Smad ubiquitination regulatory factor 2,Smurf2)参与多种生物途径,包括调节细胞周期和细胞骨架重塑。近年来,Smurf2在缺血性脑卒中的神经保护作用逐渐被熟知。研究发现,缺血性脑卒中大鼠中Smurf2的过表达可促进神经元分化进而减轻脑损伤[39]。由于转化生长因子β1受体ALK5参与缺血性脑卒中后的反应性星形胶质细胞增生和胶质瘢痕形成,而Smurf2又参与转化生长因子β受体的降解,因此Smurf2对缺血性脑卒中后星形胶质细胞的活化及突触重塑的作用备受关注。氧化三甲胺(Trimethylamine N-oxide,TMAO)是肠道微生物的代谢产物,在缺血性脑卒中后含量明显升高。TMAO通过抑制Smurf2的泛素化作用可上调ALK5的表达,进而促进反应性星形胶质细胞增生和胶质瘢痕形成,最终抑制神经功能的恢复[12]。而且SCHWEDHELM等[40]研究证实,急性缺血性脑卒中患者血浆中TMAO水平的升高与预后不良的功能结局和死亡风险密切相关。目前关于Smurf2参与 缺血性脑卒中后胶质瘢痕形成的研究相对较少,但Smurf2的升高与脑卒中诊断密切相关[41],而且Smurf2参与转化生长因子β受体的降解,可能使其在抑制星形胶质细胞活化和细胞外基质蛋白沉积方面发挥积极作用。因此Smurf2在星形胶质细胞调节缺血性脑卒中后胶质瘢痕形成中的作用值得进一步研究。 2.2.5 白细胞介素17RA/(C/EBPβ)/SIRT1信号通路 白细胞介素17及其受体白细胞介素17R在缺血性脑卒中后炎症的发生和血脑屏障的破坏中起着重要作用。白细胞介素17主要由各种免疫细胞产生,共有白细胞介素17A到白细胞介素17F共6种亚型。而星形胶质细胞是缺血性脑卒中早期产生白细胞介素17A的主要来源,在加重脑缺血性损伤方面发挥了显著作用。白细胞介素17RA在星形胶质细胞、小胶质细胞和神经元的表面均有表达,可与白细胞介素17A结合促进星形胶质细胞活化[42]。CCAAT/增强子结合蛋白β(The CCAAT/enhancer binding proteins β,C/EBPβ)是一种转录因子,在上游信号分子白细胞介素17RA的诱导下活化,可抑制沉默信息调节因子1(Silent information regulator 1,SIRT1)从而促进星形胶质细胞增殖和受损部位周围胶质瘢痕形成。总之,炎性因子白细胞介素17A在缺血性脑卒中后迅速升高,通过激活白细胞介素17RA/(C/EBPβ)/SIRT1信号通路在反应性星形胶质细胞增生中发挥作用,成为抑制胶质瘢痕形成新的潜在靶点[43]。 2.2.6 其他信号通路及分子 除上述几种主要的信号调节通路外,星形胶质细胞在胶质瘢痕形成中还涉及许多其他的信号通路及分子。A1型反应性星形胶质细胞在缺血性脑卒中中分泌大量炎性因子加速脑组织损伤,因此星形胶质细胞在炎症反应中的作用受到广泛关注。最新研究发现,星形胶质细胞表达的炎症信号分子α 和 Toll/ 白细胞介素受体结构域蛋白 1(Sterile alpha and Toll/interleukin-1 receptor motif-containing protein 1,SARM1)、空泡分类蛋白35和芳香烃在脑卒中的刺激下活化,诱导星形胶质细胞反应性增加,进一步促进神经炎症反应和胶质瘢痕的形成[44-46]。转录因子的活性是调节细胞基因表达的关键,以决定细胞在发育过程中的表型。转录调节因子Ski在缺血性脑卒中后迅速升高,通过激活有丝分裂原活化蛋白激酶(mitogen- activated protein kinase,MAPK)通路(这里指Ras-Raf-细胞外信号调节激酶1/2信号通路),促进与反应性星形胶质细胞增殖相关的蛋白表达,从而发挥促胶质瘢痕形成的作用[47]。而缺血性脑卒中后去乙酰化酶3(Sirtuins-3,SIRT3)和小胶质细胞来源的微小核糖核酸124(MicroRNA-124,miRNA-124)的增加可分别通过SIRT3/叉形头转录因子(Forkhead box O3,FOXO3a)、SIRT3/Notch1信号通路及miRNA-124/STAT3通路抑制星形胶质细胞的增殖,缩小胶质瘢痕面积[48-49]。 由此可见,星形胶质细胞形成胶质瘢痕涉及多种信号通路,主要通过调节星形胶质细胞的反应性来实现。缺血性脑卒中可直接激活星形胶质细胞活化相关信号通路,如转化生长因子β/Smad和JAK/STAT3,进而诱导星形胶质细胞反应性增高和CSPG沉积;也可以通过诱导神经炎性反应,上调炎症递质表达,从而使星形胶质细胞活化,如炎性因子白细胞介素17A下游通路和白细胞介素1β激活的JAK/STAT3信号转导。RIP1K和GSK-3β通过调节炎性因子的水平也可以间接影响星形胶质细胞活性。此外,Ski,SIRT3和miRNA可以直接影响星形胶质细胞的增殖过程。然而,这些信号通路并不是独立发挥作用的,而是多种信号通路和分子相互交织、相互影响,共同参与胶质瘢痕的形成。如何选择最佳的干预靶点仍需进一步研究,在未来明确信号通路之间的关联或许可以更好地帮助研究者筛选出有效的干预途径。 2.3 靶向星形胶质细胞胶质瘢痕形成的相关治疗 缺血性脑卒中急性期血管内溶栓和取栓等治疗虽能有效降低死亡率、减轻神经功能缺陷,但仍有约40%的患者在上述治疗后表现出较差的功能预后,提示促进脑卒中慢性期的神经再生和修复可能是改善脑卒中预后的关键[50]。基于星形胶质细胞通过反应性增生形成胶质瘢痕对缺血性脑卒中恢复期的突触再生具有抑制作用,靶向调节星形胶质细胞在胶质瘢痕形成中的关键分子和信号通路有可能成为缺血性脑卒中潜在的治疗策略[51-62],见表2。"

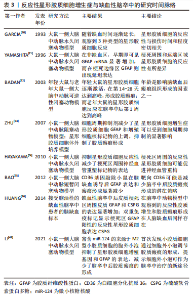
2.3.1 靶向星形胶质细胞反应性增生的药物治疗 目前,通过干预反应性星形胶质细胞增生以减弱胶质瘢痕的药物可分为3类:信号通路调节剂、细胞周期抑制剂和炎症反应调节剂。信号分子是细胞发挥特定功能的重要载体,利用药物调节特定的信号分子表达增加或减少,可以有效调节反应性星形胶质细胞的反应性,从而减轻胶质瘢痕厚度。RIP1K和GSK3β是反应性星形胶质细胞发挥促炎作用的关键调节因子。应用肿瘤抑制素(Necrostatin-1,NEC-1)及其类似物可以阻止受体相互作用蛋白(Receptor-interacting protein,RIP)复合体形成,从而特异性地抑制RIP1K的活性。在缺血性脑卒中慢性期应用该药物,不仅可显著改善梗死区周围反应性星形胶质细胞的反应性,减少胶质瘢痕厚度,而且能够促进胶质瘢痕内轴突的再生[38,51]。抑制RIP1K和GSK3β的活性还阻止了两种信号分子在胶质瘢痕形成过程中的相互作用,打破了炎症反应和瘢痕形成之间的恶性循环[37]。 褪黑素是一种神经激素,在睡眠-觉醒周期调节中发挥重要作用。但研究表明其在许多疾病中显示出抗氧化和抗炎活性[52]。最新研究发现,褪黑素在脑卒中慢性期能够促进轴突再生,这主要与其抑制星形胶质细胞的神经毒性作用和胶质瘢痕形成有关[11]。以PI3K/AKT信号通路和转化生长因子β1信号分子为靶点的药物,可有效抑制缺血性脑卒中后星形胶质细胞的活化,减少胶质瘢痕形成相关蛋白的表达,促进缺血再灌注后的突触重塑[53-54]。丙戊酸作为癫痫治疗的常用药物,最近研究发现,其可以减少星形胶质细胞活化和细胞外基质蛋白的产生,抑制缺血性脑卒中恢复期胶质瘢痕的形成,其机制可能与上调星形胶质细胞中的乙酰化组蛋白3、4和热休克蛋白70.1B的表达有关[55]。由此可见,调节星形胶质细胞反应性增生的关键信号分子在抑制胶质瘢痕形成、促进突触重塑中发挥积极作用。另外,着眼于已批准在临床治疗中使用的药物进行新的适应证和作用机制的研究,可能更具有现实意义。 反应性星形胶质细胞的大量增殖是缺血性脑卒中后形成胶质瘢痕的主要原因,通过调节星形胶质细胞的细胞周期理论上能够减少星形胶质细胞数量,从而减弱胶质瘢痕形成。最近,有研究发现,奥洛莫辛通过阻断多个细胞周期蛋白依赖激酶的方式减少缺血性脑卒中后瘢痕样星形胶质细胞的增殖,证实细胞周期抑制剂减轻胶质瘢痕形成来促进神经功能再生的合理性[56]。红景天苷在脑卒中的保护作用已有广泛研究,包括抗凋亡、抗炎以及促进神经营养因子的产生等,AKT/GSK-3β通路在其中发挥着关键作用[57-58]。而且最近研究也揭示了红景天苷通过AKT/GSK-3β通路抑制星形胶质细胞过度增殖和胶质瘢痕形成,从而增强了脑卒中后的神经可塑性[59]。上调细胞周期蛋白D1的表达也可以减弱反应性星形胶质细胞的增殖和迁移,为轴突生长提高适宜的微环境[60]。因此,细胞周期抑制剂通过调节星形胶质细胞的增殖期长短,限制其大量增殖以及在病灶周围堆积,从而在减轻缺血性脑卒中后胶质瘢痕形成中发挥积极作用。 缺血性脑卒中继发的炎症反应会诱导星形胶质细胞活化,因此减弱这种炎症反应可间接抑制胶质瘢痕的形成。苏金单抗主要用于治疗与白细胞介素17A相关的严重慢性免疫性疾病。LIU等[43]研究发现,苏金单抗在缺血性脑卒中中可通过抑制白细胞介素17RA/(C/EBPβ)/SIRT1通路,减少反应性星形胶质细胞增生和胶质瘢痕厚度,进而改善脑卒中大鼠的神经功能。此外,中草药在脑卒中后神经炎症方面的调节作用逐渐受到关注,其中的许多活性成分可以通过抑制JAK2/STAT3和GSK-3β通路抑制神经炎症风暴,减少自由基的产生,不仅保护星形胶质细胞免受凋亡的威胁,还可以减弱反应性星形胶质细胞的过度增生,从而抑制胶质瘢痕的形成[61-62]。 2.3.2 靶向反应性星形胶质细胞增生症的内源性分子治疗 缺血性脑卒中可诱导许多神经源性或血源性分子表达的改变,是星形胶质细胞形成胶质瘢痕的重要环节。神经干细胞来源的肌腱蛋白C(Tenascin-C,TNC)是一种反应性调节的细胞外基质蛋白,在缺血性脑卒中后可由星形胶质细胞大量分泌,是减少胶质瘢痕的潜在靶点;TNC缺乏后反应性星形胶质细胞增生加剧,细胞间黏附分子1表达上调刺激反应性星形胶质细胞在梗死灶周围聚集,有形成胶质瘢痕的趋势,不利于神经可塑性的恢复[63]。缺血性脑卒中诱导星形胶质细胞源性分子的表达改变,如YES相关蛋白转移至细胞核可抑制STAT3磷酸化,减少胶质瘢痕[64]。脂钙蛋白2激活JAK2/STAT3通路介导星形胶质细胞增殖和迁移,加重胶质瘢痕的形成,抑制这一靶点则可以减轻胶质瘢痕的形成[61]。血源性纤维蛋白原经受损的血脑屏障渗入脑组织,直接与瘢痕样反应性星形胶质细胞相邻。耗尽纤维蛋白原可降低星形胶质细胞的反应性和细胞外基质蛋白沉积,表明纤维蛋白原是星形胶质细胞形成胶质瘢痕的关键触发因素[65]。因此,除针对星形胶质细胞在缺血性脑卒中后发生活化、增殖和迁移等形态学改变的靶点外,调节缺血性脑卒中诱导的神经源性或血源性分子的表达同样可以降低星形胶质细胞的反应性和减少细胞外基质蛋白沉积,发挥抑制胶质瘢痕形成的作用。 2.3.3 靶向CSPG的治疗 CSPG是胶质瘢痕中阻碍神经可塑性恢复的主要化学因素,减少CSPG生成或促进其降解可有效促进体外培养的轴突生长[15]。软骨素酶ABC能够降解胶质瘢痕CSPG成分,治疗后轴突生长和功能恢复明显好转,表明其能够改善胶质瘢痕的抑制作用、促进轴突再生,是针对中枢神经系统损伤后十分有效的蛋白治疗方法[66]。SRY(性别决定区域Y)相关的高迁移率族框9(SOX9)是CSPG产生的关键调节因子,SOX9的条件性消融降低了胶质瘢痕中CSPG的含量,可促进缺血性脑卒中后修复性轴突萌发,促进神经保护和恢复[67]。缺血性脑卒中诱导组织蛋白酶B(Cathepsin B,CTSB) 从溶酶体激活并释放到细胞质中,诱导星形胶质细胞发生凋亡并引起胶质瘢痕形成。七氟醚可抑制CTSB的释放,通过稳定星形胶质细胞溶酶体膜抑制其过度激活,并降低CSPG在瘢痕内的沉积,显著减轻缺血损伤后14 d的胶质瘢痕厚度,为有效抑制瘢痕形成、发挥神经保护作用提供了新证据[68]。然而最新研究指出,CSPG的降解会导致局部环境中炎性小胶质细胞和巨噬细胞聚集,而不利于轴突再生[69]。 已有大量证据表明,星形胶质细胞在缺血性脑卒中后胶质瘢痕的形成中发挥着重要作用,见表3。以星形胶质细胞为靶点减轻胶质瘢痕形成主要有两种思路。第一,通过抑制星形胶质细胞反应性增生来减少星形胶质细胞过度增殖和聚集。已有许多药物通过调节所涉及的信号通路、抑制细胞周期蛋白以及控制神经炎症反应成功减轻了胶质瘢痕厚度,改善了神经功能结局。此外,缺血性脑卒中诱导多种内源性蛋白分子增多,也可以调控星形胶质细胞的反应性,在胶质瘢痕形成过程中发挥一定作用。第二,通过抑制细胞外基质(以CSPG为代表)的产生和在病灶周围的沉积来减少胶质瘢痕形成和促进轴突再生,见表4。然而,胶质瘢痕在缺血性脑卒中急性期限制毒性物质扩散的积极作用不容忽视,因此选择恰当的治疗时机显得尤为重要,值得进行深入探讨。"
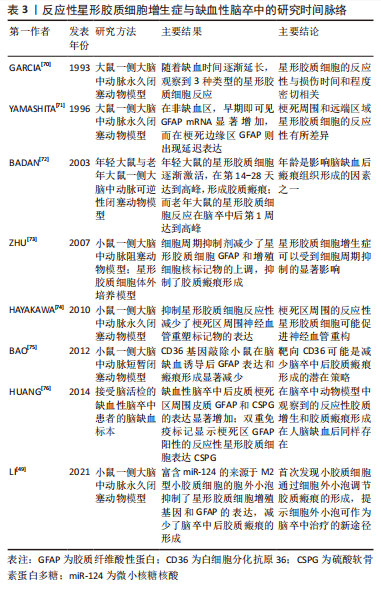
| [1] JONES SP, BAQAI K, CLEGG A, et al. Stroke in India: a systematic review of the incidence, prevalence, and case fatality. Int J Stroke. 2022;17(2):132-140. [2] ZHANG Z, SUN GY, DING S. Glial cell line-derived neurotrophic factor and focal ischemic stroke. Neurochem Res. 2021;46(10):2638-2650. [3] TANG HL, LI Y, TANG WJ, et al. Endogenous neural stem cell-induced neurogenesis after ischemic stroke: processes for brain repair and perspectives. Transl Stroke Res. 2022. doi: 10.1007/s12975-022-01078-5. [4] FAN Y, LI YK, YANG YK, et al. Chlorogenic acid promotes angiogenesis and attenuates apoptosis following cerebral ischaemia-reperfusion injury by regulating the PI3K-Akt signalling. Pharm Biol. 2022;60(1):1646-1655. [5] GAO X, ZHANG XJ, CUI LL, et al. Ginsenoside Rb1 promotes motor functional recovery and axonal regeneration in post-stroke mice through cAMP/PKA/CREB signaling pathway. Brain Res Bull. 2020;154:51-60. [6] YANG HJ, QI CX, SU F, et al. Cerebral ischemia/reperfusion injury and pharmacologic preconditioning as a means to reduce stroke-induced inflammation and damage. Neurochem Res. 2022;47(12):3598-3614. [7] LI L, ZHOU J, HAN L, et al. The specific role of reactive astrocytes in stroke. Front Cell Neurosci. 2022;16:850866. [8] WANG H, SONG G, CHUANG H, et al. Portrait of glial scar in neurological diseases. Int J Immunopathol Pharmacol. 2018;31:2058738418801406. [9] KANG J, KIM BJ, HAN MK, et al. The changing effect of blood pressure on stroke outcomes through acute to subacute stage of ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(9):2563-2568. [10] WASAN H, SINGH D, JOSHI B, et al. Dihydromyricetin alleviates cerebral ischemia-reperfusion injury by attenuating apoptosis and astrogliosis in peri-infarct corte. Neurol Res. 2022;44(5):403-414. [11] YAWOOT N, SENGKING J, WICHA P, et al. Melatonin attenuates reactive astrogliosis and glial scar formation following cerebral ischemia and reperfusion injury mediated by GSK-3 beta and RIP1K. J Cell Physiol. 2022;237(3):1818-1832. [12] SU HB, FAN SP, ZHANG LQ, et al. TMAO aggregates neurological damage following ischemic stroke by promoting reactive astrocytosis and glial scar formation via the Smurf2/ALK5 axis. Front Cell Neurosci. 2021;15:569424. [13] HEITHOFF BP, GEORGE KK, PHARES AN, et al. Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia. 2021;69(2):436-472. [14] VERKHRATSKY A, NEDERGAADR M. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos Trans R Soc Lond B Biol Sci. 2016;371(1700):20150428. [15] HE T, YANG GY, ZHANG Z. Crosstalk of astrocytes and other cells during ischemic stroke. Life (Basel). 2022;12(6):910. [16] ZAMANIAN JL, XU L, FOO LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391-6410. [17] LIDDELOW SA, GUTTENPLAN KA, LARKE LEC, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481-487. [18] DIMITROVA-SHUMKOVSKA J, KRSTANOSKI L, VEENMAN L. Diagnostic and therapeutic potential of TSPO studies regarding neurodegenerative diseases, psychiatric disorders, alcohol use disorders, traumatic brain injury, and stroke: an update. Cells. 2020;9(4):870. [19] SHEN XY, GAO ZK, HAN Y, et al. Activation and role of astrocytes in ischemic stroke. Front Cell Neurosci. 2021;15:755955. [20] WANNER IB, ANDERSON MA, SONG B, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870-12886. [21] CHAN SJ, NIU W, HAYAKAWA K, et al. Promoting neuro-supportive properties of astrocytes with epidermal growth factor hydrogels. Stem Cells Transl Med. 2019;8(12):1242-1248. [22] CHENG X, YEUNG PKK, ZHONG K, et al. Astrocytic endothelin-1 overexpression promotes neural progenitor cells proliferation and differentiation into astrocytes via the Jak2/Stat3 pathway after stroke. J Neuroinflammation. 2019;16(1):227. [23] BANITALEBI S, SKAULI N, GEISELER S, et al. Disassembly and mislocalization of AQP4 in incipient scar formation after experimental stroke. Int J Mol Sci. 2022; 23(3):1117. [24] SUN CF, LIN LY, YIN LK, et al. Acutely inhibiting AQP4 with TGN-020 improves functional outcome by attenuating edema and peri-infarct astrogliosis after cerebral ischemic. Front Immunol. 2022;13:870029. [25] SABELSTROM H, STENUDD M, REU P, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013; 342(6158):637-640. [26] HUGHES EG, KANG SH, FUKAYA M, et al. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668-676. [27] AHMED A, NAKAGAWA H, ISAKSEN TJ, et al. The effects of Bone Morphogenetic Protein 4 on adult neural stem cell proliferation, differentiation and survival in an in vitro model of ischemic stroke. Neurosci Res. 2022;183:17-29. [28] GALINDO LT, MUNDIM M, PINTO AS, et al. Chondroitin sulfate impairs neural stem cell migration through ROCK activation. Mol Neurobiol. 2018;55(4):3185-3195. [29] LORENZANA AO, LEE JK, MUI M, et al. A surviving intact branch stabilizes remaining axon architecture after injury as revealed by in vivo imaging in the mouse spinal cord. Neuron. 2015;86(4):947-954. [30] MAHAR M, CAVALLI V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19(6):323-337. [31] ZBESKO JC, NGUYEN TV, YANG T, et al. Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts. Neurobiol Dis. 2018;112:63-78. [32] ZHANG R, WU Y, XIE F, et al. RGMa mediates reactive astrogliosis and glial scar formation through TGFβ1/Smad2/3 signaling after stroke. Cell Death Differ. 2018; 25(8):1503-1516. [33] CAO H, SETO SW, BHUYAN DJ, et al. Effects of thrombin on the neurovascular unit in cerebral ischemia. Cell Mol Neurobiol. 2022;42(4):973-984. [34] SCHACHTRUP C, RYU JK, HELMRICK MJ, et al. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30(17):5843-5854. [35] RENAULT-MIHARA F, MUKAINO M, SHINOZAKI M, et al. Regulation of RhoA by STAT3 coordinates glial scar formation. J Cell Biol. 2017;216(8):2533-2550. [36] SUI Y, BIAN LG, AI QL, et al. Gastrodin inhibits inflammasome through the STAT3 signal pathways in TNA2 astrocytes and reactive astrocytes in experimentally induced cerebral ischemia in rats. Neuromolecular Med. 2019;21(3):275-286. [37] LIU J, ZHU YM, GUO Y, et al. Inhibition of GSK3β and RIP1K attenuates glial scar formation induced by ischemic stroke via reduction of inflammatory cytokine productio. Front Pharmacol. 2020;11:812. [38] ZHU ZM, LIN L, WEI C, et al. The key regulator of necroptosis, RIP1 kinase, contributes to the formation of astrogliosis and glial scar in ischemic strok. Transl Stroke Res. 2021;12(6):991-1017. [39] LIU H, SUN S, LIU B. Smurf2 exerts neuroprotective effects on cerebral ischemic injury. J Biol Chem. 2021;297(2):100537. [40] SCHWEDHELM E, VON LUCADOU M, PEINE S, et al. Trimethyllysine, vascular risk factors and outcome in acute ischemic stroke (MARK-STROKE). Amino Acids. 2021;53(4):555-561. [41] ZHANG YF, MENG LB, HAO ML, et al. Identification of co-expressed genes between atrial fibrillation and strok. Front Neurol. 2020;11:184. [42] DAI Q, LI S, LIU T, et al. Interleukin-17A-mediated alleviation of cortical astrocyte ischemic injuries affected the neurological outcome of mice with ischemic stroke. J Cell Biochem. 2019. doi: 10.1002/jcb.28429. [43] LIU SP, HUANG L, FLORES J, et al. Secukinumab attenuates reactive astrogliosis via IL-17RA/(C/EBPβ)/SIRT1 pathway in a rat model of germinal matrix hemorrhage. CNS Neurosci Ther. 2019;25(10):1151-1161. [44] 崔迈尹,付艳琼,李卓丽,等.达珀利奈抑制急性缺血性脑卒中小鼠皮层神经炎症、胶质细胞活化及SARM1表达[J].中国组织化学与细胞化学杂志, 2022,31(2):107-115. [45] YE SY, APPLE JE, REN X, et al. Microglial VPS35 deficiency regulates microglial polarization and decreases ischemic stroke-induced damage in the cortex. J Neuroinflammation. 2019;16(1):235. [46] CHEN WC, CHANG LH, HUANG SS, et al. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflammation. 2019;16(1):187. [47] ZHAO X, ZHOU KS, LI ZH, et al. Knockdown of Ski decreased the reactive astrocytes proliferation in vitro induced by oxygen-glucose deprivation/reoxygenatio. J Cell Biochem. 2018;119(6):4548-4558. [48] YANG X, GENG KY, ZHANG JF, et al. Sirt3 mediates the inhibitory effect of adjudin on astrocyte activation and glial scar formation following ischemic stroke. Front Pharmacol. 2017;8:943. [49] LI Z, SONG Y, HE T, et al. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics. 2021;11(3):1232-1248. [50] YUAN YJ, LIU LJ, DU Y, et al. P-hydroxy benzaldehyde revitalizes the microenvironment of peri-infarct cortex in rats after cerebral ischemia-reperfusion. Phytomedicine. 2022;105:154379. [51] LI W, LIU J, CHEN JR, et al. Neuroprotective effects of DTIO, a novel analog of Nec-1, in acute and chronic stages after ischemic stroke. Neuroscience. 2018; 390: 12-29. [52] CHITIMUS DM, POPESCU MR, VOICULESCU SE, et al. Melatonin’s impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecule. 2020;10(9):1211. [53] LU T, LI H, ZHOU Y, et al. Neuroprotective effects of alisol A 24-acetate on cerebral ischaemia-reperfusion injury are mediated by regulating the PI3K/AKT pathway. J Neuroinflammation. 2022;19(1):37. [54] LUO D, ZHANG Y, YUAN X, et al. Oleoylethanolamide inhibits glial activation via moudulating PPARα and promotes motor function recovery after brain ischemi. Pharmacol Res. 2019;141:530-540. [55] GAO X, ZEB S, HE YY, et al. Valproic acid inhibits glial scar formation after ischemic stroke. Pharmacology. 2022;107(5-6):263-280. [56] YEW WP, DJUKIC ND, JAYASEELAN JSP, et al. Differential effects of the cell cycle inhibitor, olomoucine, on functional recovery and on responses of peri-infarct microglia and astrocytes following photothrombotic stroke in rats. J Neuroinflammation. 2021;18(1):168. [57] ZHANG X, DU QM, YANG Y, et al. Salidroside alleviates ischemic brain injury in mice with ischemic stroke through regulating BDNK mediated PI3K/Akt pathway. Biochem Pharmacol. 2018;156:99-108. [58] WEI YC, HONG HM, ZHANG XQ, et al. Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral ischemia in rats. Inflammation. 2017; 40(4):1297-1309. [59] DONG C, WEN S, ZHAO S, et al. Salidroside inhibits reactive astrogliosis and glial dcar formation in late cerebral ischemia via the Akt/GSK-3β pathway. Neurochem Res. 2021;46(4):755-769. [60] OU-YANG L, LIU Y, WANG BY, et al. Carnosine suppresses oxygen-glucose deprivation/recovery-induced proliferation and migration of reactive astrocytes of rats in vitro. Acta Pharmacol Sin. 2018;39(1):24-34. [61] ZHANG Y, LIU J, YAO M, et al. Sailuotong capsule prevents the cerebral ischaemia-induced neuroinflammation and impairment of recognition memory through inhibition of LCN2 expression. Oxid Med Cell Longev. 2019; 2019:8416105. [62] GHAZAVI H, SHIRZAD S, FOROUZANFAR F, et al. The role of resveratrol as a natural modulator in glia activation in experimental models of stroke. Avicenna J Phytomed. 2020;10(6):557-573. [63] DZYUBENKO E, MANRIQUE-CASTANO D, PILLATH-EILERS M, et al. Tenascin-C restricts reactive astrogliosis in the ischemic brain. Matrix Biol. 2022;110:1-15. [64] HUANG L, LI S, DAI Q, et al. Astrocytic Yes-associated protein attenuates cerebral ischemia-induced brain injury by regulating signal transducer and activator of transcription 3 signaling. Exp Neurol. 2020;333:113431. [65] CONFORTI P, MEZEY S, NATH S, et al. Fibrinogen regulates lesion border-forming reactive astrocyte properties after vascular damage. Glia. 2022;70(7):1251-1266. [66] HETTIARATCHI MH, O’MEARA MJ, TEAL CJ, et al. Local delivery of stabilized chondroitinase ABC degrades chondroitin sulfate proteoglycans in stroke-injured rat brains. J Control Release. 2019;297:14-25. [67] XU X, BASS B, MCKILLOP WM, et al. Sox9 knockout mice have improved recovery following stroke. Exp Neurol. 2018;303:59-71. [68] ZHU YM, GAO X, NI Y, et al. Sevoflurane postconditioning attenuates reactive astrogliosis and glial scar formation after ischemia-reperfusion brain injury. Neuroscience. 2017;356:125-141. [69] SUN XM, Liu HQ, TAN Z, et al. Remodeling microenvironment for endogenous repair through precise modulation of chondroitin sulfate proteoglycans following spinal cord injury. Small. 2022. doi:1002/smll.202205012. [70] GARCIA JH, YOSHIDA Y, CHEN H, et al. Progression from ischemic-injury to infarct following middle cerebral-artery occlusion in the rat. Am J Pathol. 1993; 142(2):623-635. [71] YAMASHITA K, VOGEL P, FRITZE K, et al. Monitoring the temporal and spatial activation pattern of astrocytes in focal cerebral ischemia using in situ hybridization to GFAP mRNA: comparison with sgp-2 and hsp70 mRNA and the effect of glutamate receptor antagonist. Brain Res. 1996;735(2):285-297. [72] BADAN I, BUCHHOLD B, HAMM A, et al. Accelerated glial reactivity with reduced to stroke in aged rats correlates functional recover. J Cereb Blood Flow Metab. 2003;23(7):845-854. [73] ZHU Z, ZHANG Q, YU Z, et al. Inhibiting cell cycle progression reduces reactive astrogliosis initiated by scratch injury in vitro and by cerebral ischemia in vivo. Glia. 2007;55(5):546-558. [74] HAYAKAWA K, NAKANO T, IRIE K, et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010;30(4):871-882. [75] BAO Y, QIN LY, KIM E, et al. CD36 is involved in astrocyte activation and astroglial scar formation. J Cereb Blood Flow Metab. 2012;32(8):1567-1577. [76] HUANG LJ, WU ZB, ZHUGE QC, et al. Glial scar formation occurs in the human brain after ischemic stroke. Int J Med Sci. 2014;11(4):344-348. [77] TRAN AP, SUNDAR S, YU MG, et al. Modulation of receptor protein tyrosine phosphatase sigma increases chondroitin sulfate proteoglycan degradation through cathepsin B secretion to enhance axon outgrowt. J Neurosci. 2018; 38(23):5399-5414. [78] LUO FC, WANG JP, ZHANG Z, et al. Inhibition of CSPG receptor PTPs promotes migration of newly born neuroblasts, axonal sprouting, and recovery from stroke. Cell Rep. 2022;40(4):111137. |
| [1] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [2] | Shen Feiyan, Yao Jixiang, Su Shanshan, Zhao Zhongmin, Tang Weidong. Knockdown of circRNA WD repeat containing protein 1 inhibits proliferation and induces apoptosis of chondrocytes in knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 499-504. |
| [3] | Wu Tian, Zhao Yue, Hu Rong. Effect of nanobubbles carrying double antibodies on the proliferation of ovarian cancer cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 341-346. |
| [4] | Xu Yinghua, Liu Jing, You Quan, Wen Zhihao, Gao Lu. Effect of neodymium-doped:yttrium aluminum perovskite laser combined with two kinds of remineralizers on remineralization of early enamel caries [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 360-365. |
| [5] | Li Lisi, Zhang Chengdong, Li Xiaolong, Ye Ziyu, Pu Chao, Yang Zaijun, Shi Feng, Xiao Dongqin. Growth differentiation factor-5 modified by bisphosphonate promotes osteogenic differentiation of MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 373-379. |
| [6] | Wang Xinyi, Xie Xianrui, Chen Yujie, Wang Xiaoyu, Xu Xiaoqing, Shen Yihong, Mo Xiumei. Electrospun nanofiber scaffolds for soft and hard tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 426-432. |
| [7] | Gao Xueyu, Zhang Wentao, Sun Tianze, Zhang Jing, Li Zhonghai. Application of metal ions in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 439-444. |
| [8] | Chen Pinrui, Pei Xibo, Xue Yiyuan. Function and advantages of magnetically responsive hydrogel in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 452-457. |
| [9] | Long Zhirui, Huang Lei, Xiao Fang, Wang Lin, Wang Xiaobei. Characteristics of hydrogel microspheres in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 472-478. |
| [10] | Xu Jing, Lyu Huixin, Bao Xin, Zhang Yi, Wang Yihan, Zhou Yanmin. Application of near infrared responsive hydrogels in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 486-492. |
| [11] | Sun Yuan, Wang Qingbo, Pi Yihua, Lu Chunmin, Xu Chuanyi, Zhang Yan. Effects of early and late aerobic exercise on right heart failure induced by monocrotaline in rats with pulmonary hypertension [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 177-185. |
| [12] | Liu Baofang, Xu Bin, Chen Lei. Pueraria decoction in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 193-199. |
| [13] | Ran Lei, Han Haihui, Xu Bo, Wang Jianye, Shen Jun, Xiao Lianbo, Shi Qi. Molecular docking analysis of the anti-inflammatory mechanism of Cibotium barometz and Epimedium for rheumatoid arthritis: animal experiment validation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 208-215. |
| [14] | Wang Qian, Lu Ziang, Li Lihe, Lyu Chaoliang, Wang Meng, Zhang Cunxin. Sinomenine effectively inhibits interleukin-1beta-induced apoptosis in nucleus pulposus cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 224-230. |
| [15] | Long Yi, Yang Jiaming, Ye Hua, Zhong Yanbiao, Wang Maoyuan. Extracellular vesicles in sarcopenic obesity: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 315-320. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

