Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (2): 177-185.doi: 10.12307/2023.851
Previous Articles Next Articles
Effects of early and late aerobic exercise on right heart failure induced by monocrotaline in rats with pulmonary hypertension
Sun Yuan1, Wang Qingbo2, Pi Yihua2, Lu Chunmin2, Xu Chuanyi2, Zhang Yan2
- 1Lianyungang Normal College, Lianyungang 222006, Jiangsu Province, China; 2Department of Sports, Guangxi University of Chinese Medicine, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Received:2022-10-24Revised:2022-11-30Online:2024-01-18Published:2023-06-29 -
Contact:Zhang Yan, Master, Associate professor, Department of Sports, Guangxi University of Chinese Medicine, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Sun Yuan, Master, Lecturer, Lianyungang Normal College, Lianyungang 222006, Jiangsu Province, China -
Supported by:Guangxi Provincial Education Science "13th Five-Year Plan" Project, No. 2017C386 (to ZY)
CLC Number:
Cite this article
Sun Yuan, Wang Qingbo, Pi Yihua, Lu Chunmin, Xu Chuanyi, Zhang Yan. Effects of early and late aerobic exercise on right heart failure induced by monocrotaline in rats with pulmonary hypertension[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 177-185.
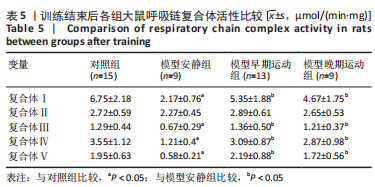
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
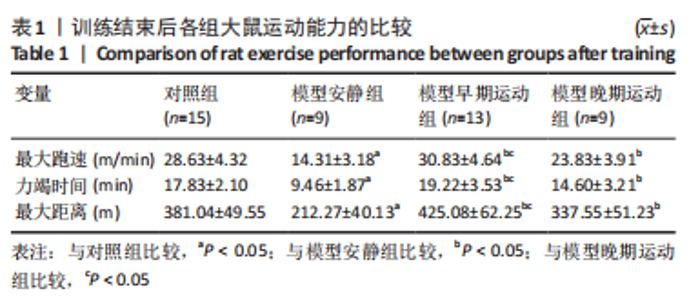
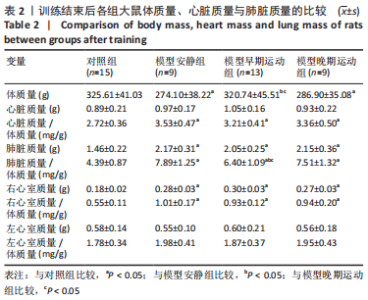
训练结束后,与对照组比较,模型安静组大鼠体质量下降(P < 0.05),心脏质量/体质量、肺脏质量、肺脏质量/体质量、右心室质量和右心室质量/体质量增加(P < 0.05);模型早期、晚期运动组大鼠心脏质量/体质量、肺脏质量、肺脏质量/体质量、右心室质量和右心室质量/体质量增加(P < 0.05),模型晚期运动组大鼠体质量下降。与模型安静组比较,模型早期运动组大鼠体质量增加(P < 0.05),肺脏质量/体质量下降(P < 0.05);模型晚期运动组各参数无明显变化(P > 0.05)。与模型晚期运动组比较,模型早期运动组大鼠体质量增加(P < 0.05),肺脏质量/体质量下降(P < 0.05)。 2.4 各组大鼠超声心动参数的比较 见表3。 "
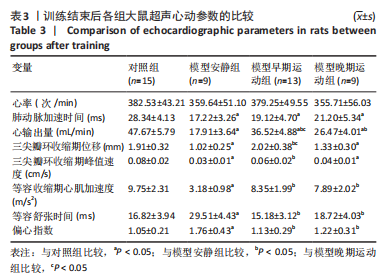
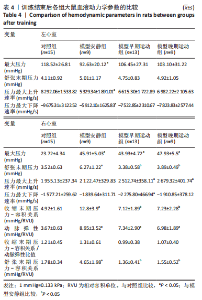
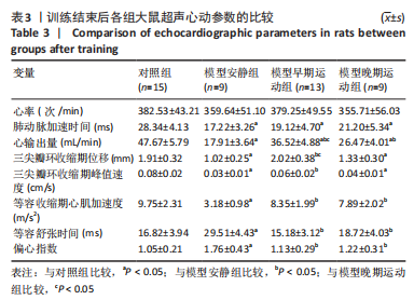
训练结束后,与对照组比较,模型安静组大鼠肺动脉加速时间、心输出量、三尖瓣环收缩期位移、三尖瓣环收缩期峰值速度和等容收缩期心肌加速度下降(P < 0.05),等容舒张时间和偏心指数升高(P < 0.05);模型早期运动组大鼠肺动脉加速时间和心输出量下降(P < 0.05);模型晚期运动组大鼠肺动脉加速时间、心输出量、三尖瓣环收缩期位移和三尖瓣环收缩期峰值速度下降(P < 0.05)。与模型安静组比较,模型早期运动组大鼠心输出量、三尖瓣环收缩期位移、三尖瓣环收缩期峰值速度和等容收缩期心肌加速度升高(P < 0.05),等容舒张时间和偏心指数下降(P < 0.05);模型晚期运动组大鼠心输出量和等容收缩期心肌加速度升高(P < 0.05),等容舒张时间和偏心指数下降(P < 0.05)。与模型晚期运动组比较,模型早期运动组大鼠心输出量和三尖瓣环收缩期位移增加(P < 0.05)。 2.5 各组大鼠血液动力学参数的比较 见表4。"
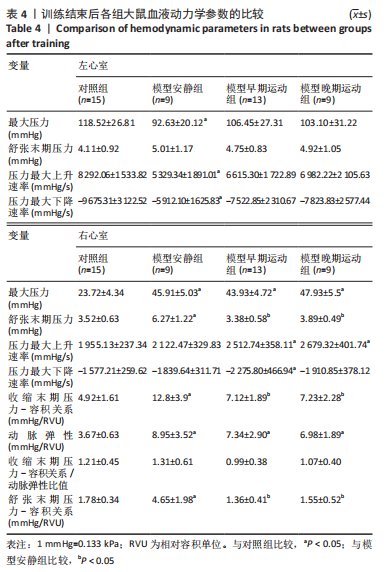
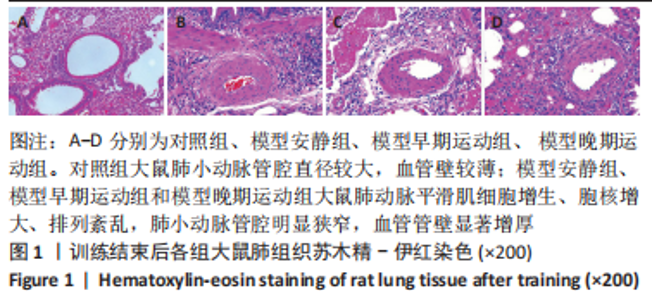
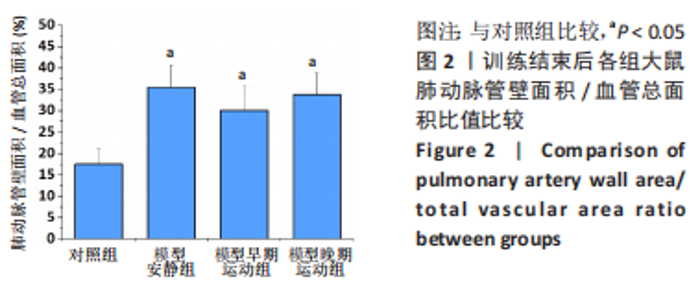
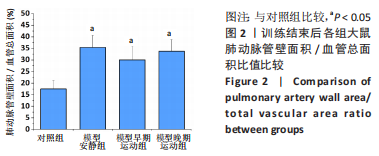
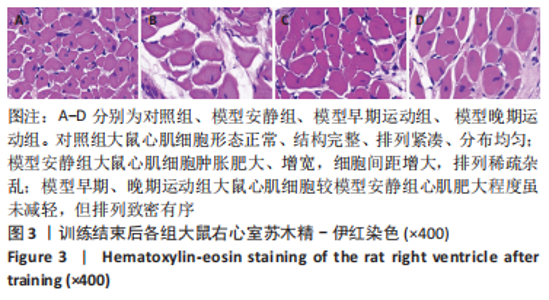
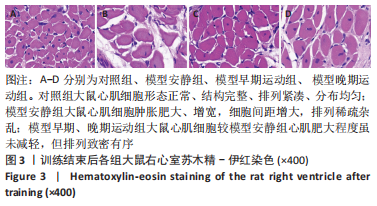
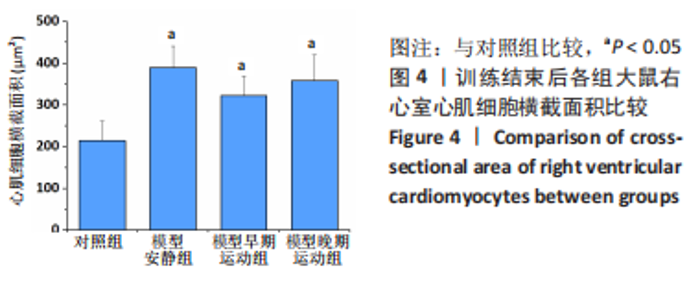
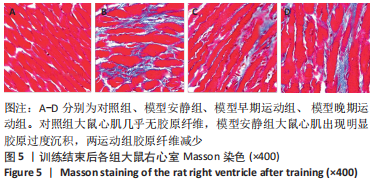
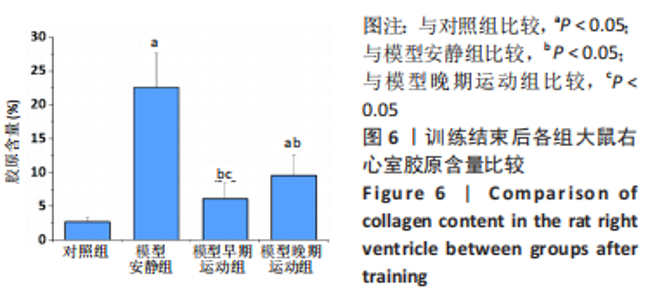
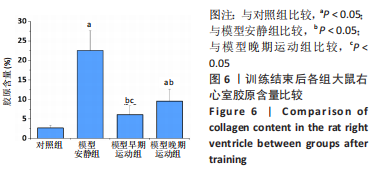
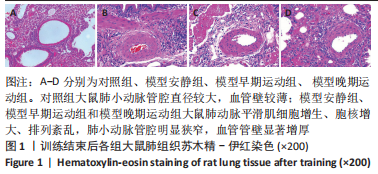
训练结束后,与对照组比较,模型安静组大鼠左心室最大压力、压力最大上升速率和压力最大下降速率下降(P < 0.05),右心室最大压力、舒张末期压力、收缩末期压力-容积关系、动脉弹性和舒张末期压力-容积关系升高(P < 0.05);模型早期运动组大鼠右心室最大压力、压力最大上升速率、压力最大下降速率和动脉弹性升高(P < 0.05);模型晚期运动组大鼠右心室最大压力、压力最大上升速率和动脉弹性升高(P < 0.05)。与模型安静组比较,模型早期运动组和模型晚期运动组右心室舒张末期压力、收缩末期压力-容积关系和舒张末期压力-容积关系下降(P < 0.05)。与模型晚期运动组比较,模型早期运动组左右心室各参数无明显变化(P > 0.05)。 2.6 各组大鼠肺脏和右心室组织病理学变化 肺小动脉苏木精-伊红染色:对照组大鼠肺小动脉管腔直径较大,血管壁较薄;模型安静组、模型早期运动组和模型晚期运动组大鼠肺动脉平滑肌细胞增生、胞核增大、排列紊乱,肺小动脉管腔明显狭窄,血管管壁显著增厚,见图1。与对照组比较,模型安静组、模型早期运动组和模型晚期运动组大鼠肺动脉管壁面积/血管总面积比值均显著性升高(P < 0.05),各模型组间比较无显著性意义(P > 0.05),见图2。"
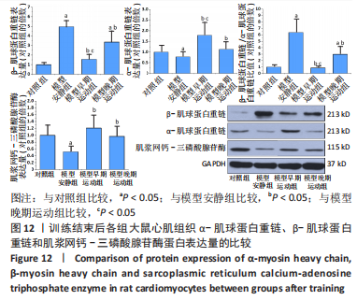
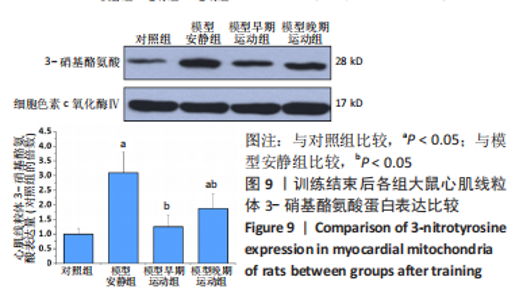
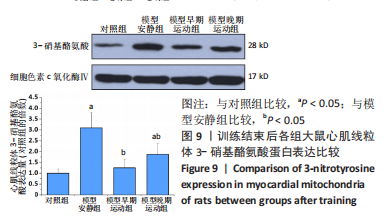
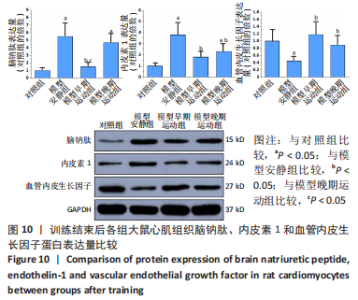
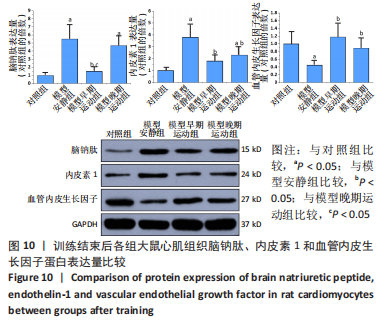
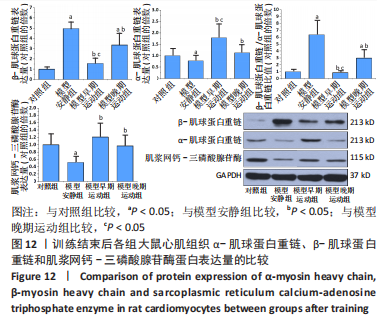
心脏收缩蛋白:与对照组比较,模型安静组β-肌球蛋白重链蛋白表达量和β-肌球蛋白重链/α-肌球蛋白重链比值升高(P < 0.05),α-肌球蛋白重链和肌浆网钙-三磷酸腺苷酶蛋白表达量下降(P < 0.05)。与模型安静组比较,模型早期、晚期运动组α-肌球蛋白重链和肌浆网钙-三磷酸腺苷酶蛋白表达量升高(P < 0.05),β-肌球蛋白重链蛋白表达量及β-肌球蛋白重链/α-肌球蛋白重链比值下降(P < 0.05)。与模型晚期运动组比较,模型早期运动组α-肌球蛋白重链蛋白表达量升高(P < 0.05),β-肌球蛋白重链蛋白表达量和β-肌球蛋白重链/α-肌球蛋白重链比值下降(P < 0.05),见图12。"
| [1] 杨媛华.《中国肺动脉高压诊断与治疗指南(2021版)》解读——肺动脉高压的诊断[J]. 中国实用内科杂志,2021,41(8):696-699. [2] KLINGER JR, ELLIOTT CG, LEVINE DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the chest guideline and expert panel report. Chest. 2019;155(3):565-586. [3] BENJAMIN N, MARRA AM, EICHSTAEDT C, et al. Exercise training and rehabilitation in pulmonary hypertension. Heart Fail Clin. 2018;14(3):425-430. [4] SANTOS-LOZANO A, FIUZA-LUCES C, FERNÁNDEZ-MORENO D, et al. Exercise benefits in pulmonary hypertension. J Am Coll Cardiol. 2019;73(22):2906-2907. [5] CASSADY SJ, RAMANI GV. Right heart failure in pulmonary hypertension. Cardiol Clin. 2020;38(2):243-255. [6] GHIO S, RAINERI C, SCELSI L, et al. Pulmonary hypertension and right ventricular remodeling in HFpEF and HFrEF. Heart Fail Rev. 2020;25(1):85-91. [7] CHIABRANDO JG, FEOLA M. Exercise and adverse ventricular remodeling: the cornerstone of heart failure. Minerva Cardiol Angiol. 2021;69(4):435-437. [8] HANDOKO ML, DE MAN FS, HAPPÉ CM, et al. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation. 2009;120(1):42-49. [9] MEYRICK B, GAMBLE W, REID L. Development of crotalaria pulmonary hypertension: hemodynamic and structural study. Am J Physiol. 1980;239(5):H692-702. [10] 张艳,何瑞波,王庆博,等.不同负荷量有氧运动对肥胖大鼠骨骼肌炎症反应和胰岛素信号途径的影响及机制[J].中国组织工程研究,2023,27(8):1237-1244. [11] XU X, CHEN C, LU WJ, et al. Pyrroloquinoline quinone can prevent chronic heart failure by regulating mitochondrial function. Cardiovasc Diagn Ther. 2020;10(3): 453-469. [12] 张昊,王伟伟,薛过,等.运动训练通过诱导自噬、抑制凋亡改善老龄大鼠心脏功能[J].中国病理生理杂志,2014,30(1):11-17. [13] 王增喜,李洁,王悦.高强度间歇训练对大鼠心肌线粒体呼吸链复合体活性的影响[J].中国运动医学杂志,2018,37(4):315-322. [14] BOGAARD HJ, NATARAJAN R, HENDERSON SC, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009; 120(20):1951-1960. [15] WEISSMANN N, PETERS DM, KLÖPPING C, et al. Structural and functional prevention of hypoxia-induced pulmonary hypertension by individualized exercise training in mice. Am J Physiol Lung Cell Mol Physiol. 2014;306(11):L986-995. [16] SAEED S, HOLM H, NILSSON PM. Ventricular-arterial coupling: definition, pathophysiology and therapeutic targets in cardiovascular disease. Expert Rev Cardiovasc Ther. 2021;19(8):753-761. [17] SPRUIJT OA, DE MAN FS, GROEPENHOFF H, et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2015;191(9):1050-1057. [18] EHLKEN N, LICHTBLAU M, KLOSE H, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J. 2016;37(1):35-44. [19] BLANCO I, TORRES-CASTRO R, BARBERÀ JA. Exercise tolerance in pulmonary hypertension. Arch Bronconeumol. 2022;58(5):388-389. [20] O’CALLAGHAN DS, HUMBERT M. A critical analysis of survival in pulmonary arterial hypertension. Eur Respir Rev. 2012;21(125):218-222. [21] GRÜNIG E, MACKENZIE A, PEACOCK AJ, et al. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J. 2021;42(23):2284-2295. [22] ROIBAL PRAVIO J, BARGE CABALLERO E, BARBEITO CAAMAÑO C, et al. Determinants of maximal oxygen uptake in patients with heart failure. ESC Heart Fail. 2021;8(3):2002-2008. [23] KASHIMURA O, SAKAI A, YANAGIDAIRA Y. Effects of exercise-training on hypoxia and angiotensin II-induced pulmonary vasoconstrictions. Acta Physiol Scand. 1995;155(3):291-295. [24] STĘPNOWSKA E, LEWICKA E, DĄBROWSKA-KUGACKA A, et al. Prognostic factors in pulmonary arterial hypertension: Literature review. Adv Clin Exp Med. 2017; 26(3):549-553. [25] ROGNMO Ø, MOHOLDT T, BAKKEN H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126(12):1436-1440. [26] HUSSAIN SR, MACALUSO A, PEARSON SJ. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol Rev. 2016;24(6):273-281. [27] GONZÁLEZ A, SCHELBERT EB, DÍEZ J, et al. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018; 71(15):1696-1706. [28] LIAO Z, LI D, CHEN Y, et al. Early moderate exercise benefits myocardial infarction healing via improvement of inflammation and ventricular remodelling in rats. J Cell Mol Med. 2019; 23(12):8328-8342. [29] 王雪芳,谢萍.肺动脉高压与免疫炎症[J].临床心血管病杂志,2019,35(5): 397-401. [30] KUMARI R, KUMAR S, AHMAD MK, et al. TNF-α/IL-10 ratio: an independent predictor for coronary artery disease in North Indian population. Diabetes Metab Syndr. 2018; 12(3):221-225. [31] NOVOYATLEVA T, SCHYMURA Y, JANSSEN W, et al. Deletion of Fn14 receptor protects from right heart fibrosis and dysfunction. Basic Res Cardiol. 2013;108(2):e325. [32] FALCÃO-PIRES I, GONÇALVES N, HENRIQUES-COELHO T, et al. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;296(6): H2007-2014. [33] LOURENÇO AP, RONCON-ALBUQUERQUE R JR, BRÁS-SILVA C, et al. Myocardial dysfunction and neurohumoral activation without remodeling in left ventricle of monocrotaline-induced pulmonary hypertensive rats. Am J Physiol Heart Circ Physiol. 2006;291(4):H1587-1594. [34] MIAO H, QIU F, ZHU L, et al. Novel angiogenesis strategy to ameliorate pulmonary hypertension. J Thorac Cardiovasc Surg. 2021;161(6):e417-434. [35] MORKIN E. Control of cardiac myosin heavy chain gene expression. Microsc Res Tech. 2000;50(6):522-531. [36] JAMES J, HOR K, MOGA MA, et al. Effects of myosin heavy chain manipulation in experimental heart failure. J Mol Cell Cardiol. 2010;48(5):999-1006. [37] KORTE FS, HERRON TJ, ROVETTO MJ, et al. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am J Physiol Heart Circ Physiol. 2005;289(2):H801-812. [38] ZHIHAO L, JINGYU N, LAN L, et al. SERCA2a: a key protein in the Ca2+ cycle of the heart failure. Heart Fail Rev. 2020;25(3):523-535. [39] HSU CH, ROAN JN, FANG SY, et al. Transplantation of viable mitochondria improves right ventricular performance and pulmonary artery remodeling in rats with pulmonary arterial hypertension. J Thorac Cardiovasc Surg. 2022;163(5):e361-373. [40] PEOPLES JN, SARAF A, GHAZAL N, et al. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. 2019;51(12):1-13. [41] JIANG M, ZHAO XM, JIANG ZS, et al. Protein tyrosine nitration in atherosclerotic endothelial dysfunction. Clin Chim Acta. 2022;529:34-41. |
| [1] | Chen Simin, Hu Yingjun, Yan Wenrui, Ji Le, Shao Mengli, Sun Ze, Zheng Hongxing, Qi Shanshan. Establishment and evaluation of a streptozotocin-induced diabetic encephalopathy rat model [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 237-241. |
| [2] | Wang Jingfeng, Wen Dengtai, Wang Shijie, Gao Yinghui. Atg-mediated autophagy, exercise and skeletal muscle aging [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 295-301. |
| [3] | Long Yi, Yang Jiaming, Ye Hua, Zhong Yanbiao, Wang Maoyuan. Extracellular vesicles in sarcopenic obesity: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 315-320. |
| [4] | Yang Ting, Ding Zhibin, Jiang Nan, Han Hongxia, Hou Miaomiao, Ma Cungen, Song Lijuan, Li Xinyi. Astrocytes regulate glial scar formation in cerebral ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 131-138. |
| [5] | Ma Suilu, He Zhijun, Liu Tao, Li Yan, He Yuanxu, He Bo, Wang Weiwei, Wei Xiaotao. Traditional Chinese medicine monomer in the prevention and treatment of flap necrosis by regulating “autophagy” [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 153-158. |
| [6] | Guo Shuhui, Yang Ye, Jiang Yangyang, Xu Jianwen. Screening and validation of neurogenic bladder miRNA-mRNA regulatory network [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-8. |
| [7] | Fang Xingyan, Tian Zhenli, Zhao Zheyi, Wen Ping, Xie Tingting. Effects of sodium arsenite on human umbilical vein endothelial cell injury and sphingosine kinases 1/sphingosine 1-phosphate signaling axis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-7. |
| [8] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [9] | Tang Liang, Li Xiheng, Niu Ruijuan, Li Xinyue, Zou Xinying, Mao Tianjiao, Li Jiang. Naringin regulates the function of RAW264.7 macrophages to affect the osteogenic differentiation of MC-3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1205-1210. |
| [10] | Ruan Ling, Wang Guanghua, Wu Rongping, Jin Zhan, Lyu Zhenqing, Zhang Nan, Li Shoubang. Correlation between exercise intensity and lipid metabolism disorder and oxidative stress in a high-diet rat model [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1149-1155. |
| [11] | Zhang Yan, He Ruibo, Wang Qingbo, Pi Yihua, Lu Chunmin, Xu Chuanyi, Ma Gang, Peng Peng. Effects of aerobic exercises with different load volumes on inflammatory response and insulin signaling pathway of skeletal muscle in obese rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1237-1244. |
| [12] | Wang Ji, Zhang Min, Yang Zhongya, Zhang Long. A review of physical activity intervention in type 2 diabetes mellitus with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1272-1277. |
| [13] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [14] | Liang Jiaqi, Liu Hengxu, Yang Jinxin, Yang Yi, Deng Xuhui, Tan Mingjian, Luo Jiong. Health benefit relationship between exercise and intestinal bacteria [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1292-1299. |
| [15] | Gao Yu, Han Jiahui, Ge Xin. Immunoinflammatory microenvironment after spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1300-1305. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||