Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (29): 4745-4750.doi: 10.12307/2023.675
Previous Articles Next Articles
Research advances in animal models of ankle osteoarthritis
Zhou Guangzhi1, Tai Dongxu2
- 1Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China; 2Second Department of Orthopedics and Trauma, Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China
-
Received:2022-08-22Accepted:2022-10-12Online:2023-10-18Published:2022-12-02 -
Contact:Tai Dongxu, Doctoral candidate, Professor, Chief physician, Second Department of Orthopedics and Trauma, Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China -
About author:Zhou Guangzhi, Master candidate, Physician, Liaoning University of Traditional Chinese Medicine, Shenyang 110032, Liaoning Province, China
CLC Number:
Cite this article
Zhou Guangzhi, Tai Dongxu. Research advances in animal models of ankle osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(29): 4745-4750.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
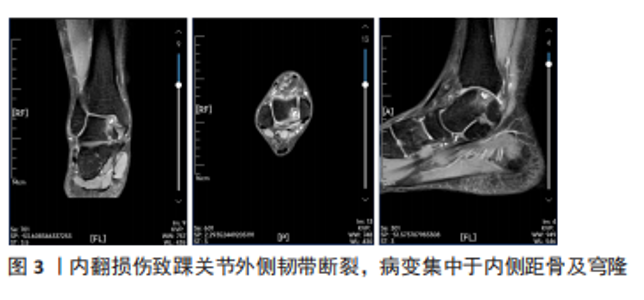
2.1 踝关节骨关节炎病因 2.1.1 创伤性关节炎 创伤性骨关节炎是踝关节骨关节炎最常见的形式[7]。危险因素包括:Weber C型骨折、内外踝骨折、关节脱位、体质量指数、年龄、术后时间,在具有3个或以上危险因素的患者,在术后12-22年中发生创伤性骨关节炎的概率为60%-70%[8]。创伤性骨关节炎的发生是多种因素作用的结果。首先严重的关节内骨折往往影响关节软骨的再生,踝关节中任何部位的软骨损伤都是踝关节创伤性骨关节炎发展的独立危险因素[9];其次创伤后生物力学的改变可能会改变踝关节力学负荷分布,导致关节软骨损伤[10]。慢性踝关节不稳如何导致创伤性骨关节炎的明确机制尚不清楚,机械因素可能在疾病过程中起一定作用[11],在严重的踝关节扭伤时,内侧距骨及穹隆软骨常常受累较大,损伤较外侧病变更深(图3),这种损伤机制可以距骨软骨的解剖学分布解释。但约95%的严重踝关节扭伤导致距骨软骨损伤中,仅半数的患者会出现骨关节炎[12],因此仅靠关节不稳并不能完全解释创伤性骨关节炎的发生率。"
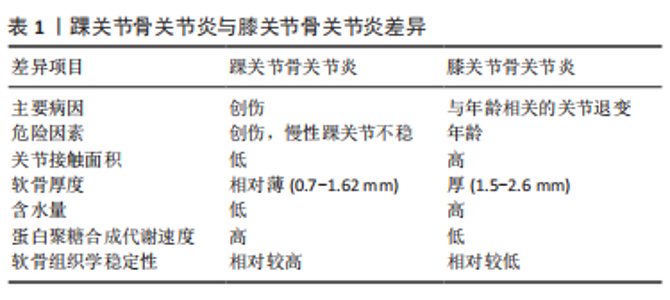
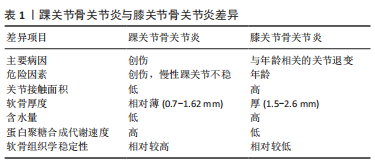
2.1.2 原发性骨关节炎 生理状态下的软骨组织由单一类型的软骨细胞及细胞外基质组成,踝关节局部软骨组织结构特殊,踝关节单位厚度的软骨细胞密度[(41±34)×103/mg]比膝关节软骨[(28±26)×103/mg]高48%[4]。虽然临床中踝关节骨关节炎绝大多数属于创伤性骨关节炎,但与膝关节相比,踝关节不容易罹患软骨退行性疾病(即原发性骨关节炎)[7]。而原发性骨关节发病机制的经典观点是与年龄相关的关节退行性变,严重的关节软骨表面磨损以及软骨下骨的硬化乃至关节力学的改变是其病理学变化的主要的特征[13]。在JALEEL等[14]关于踝关节骨关节炎的流行病学调查中,仅有7%的患者患有原发性踝关节骨关节炎。由于踝关节软骨组织独特的组织学结构,如软骨厚度相对较薄、软骨刚性较高,因此在组织学上稳定性更高[4]。同时踝关节软骨的细胞外基质特性也使得原发性骨关节炎的发生较少[13],见表1。"
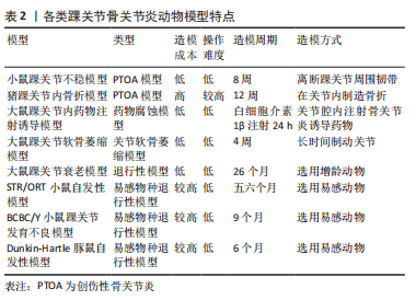
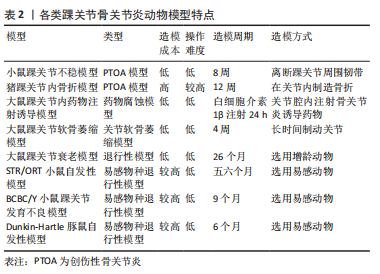
2.2 踝关节骨关节炎动物模型 2.2.1 创伤性骨关节炎动物模型 (1)小鼠踝关节不稳致骨关节炎模型:小鼠踝关节骨关节炎的3种不稳定模型包括离断内踝韧带的内侧不稳模型、离断外踝韧带的外踝不稳模型及内外踝韧带皆离断的不稳模型。因内踝韧带对于维持胫距关节稳定的重要性,在内侧不稳模型中,损伤主要累及胫距关节,而距下关节受累较少;相反,在外侧不稳模型中,距下关节主要受累较为明显,而胫距关节受到的损伤较少[15]。 在踝关节外侧韧带中,目前缺乏小鼠距腓前韧带(ATFL)与人类的距腓前韧带功能(限制距骨前移及内翻,踝关节主要稳定装置)是否一致的研究[16]。跟腓韧带(CFL)是距下关节的主要稳定器,并且是小鼠踝关节外侧唯一坚固的韧带,在外踝不稳模型中,一般切除外侧韧带(包括距腓前韧带,跟腓韧带)就可导致距下关节的严重不稳定,是踝关节骨关节炎模型最常用,较简便的动物造模方式。 在内侧不稳模型中,末端转移酶(terminal deoxyribonucleotidyl transferase,TdT)介导的脱氧尿嘧啶核苷三磷酸(dUTP)和Adamts5(ADAM Metallopeptidase With Thrombospondin Type 1 Motif 5)表达在骨关节炎早期和中期均得到增强,基质金属蛋白酶13(MMP13)的表达在骨关节炎发展过程中也呈逐渐升高的态势[17]。在人类中,距腓前韧带或跟腓韧带断裂是踝关节骨关节炎的常见原因;然而,单侧不稳与胫距关节或距下关节骨关节炎之间的关系还未完全阐明。需要对人类和动物模型的进一步的研究[18]。 (2)猪踝关节内骨折模型:该模型用于研究关节内骨折诱导的创伤性骨关节炎以及因关节表面损伤而引起的慢性踝关节力学改变的影响[19]。GOETZ等[20]将尤卡坦迷你猪的胫骨远端采用开放骨折手术,并使用骨板内固定进行修复,术后进行关节液分析、影像学及力学分析,12周时处死动物。检测了相关炎性细胞因子的表达,进行骨软骨组织学分析。到术后12周,所有骨折均愈合,肢体负荷恢复正常;滑液中的炎性细胞因子浓度,包括肿瘤坏死因子α、白细胞介素1β、白细胞介素6和白细胞介素8在骨折后2周内短暂升高;与解剖学重建的关节相比,关节不协调关节的组织学评分更差。该模型复制了人类关节内骨折的关键特征,包括手术稳定、炎症反应和进展为骨关节炎软骨变性,从而为将有希望的治疗方案转化为临床实践提供了一个潜在的有用模型。 2.2.2 非创伤性动物模型 (1)大鼠踝关节内药物注射诱导骨关节炎模型:此方法主要将致炎或有毒化合物注射至关节腔内,通过腐蚀软骨组织的细胞外基质从而诱导骨关节炎发生。与膝关节常用的木瓜蛋白酶不同,踝关节并未有使用该药物诱导踝骨关节炎发生的报道,可能与踝关节与膝关节软骨组织结构不同有关。SCOTT等[21]将白细胞介素1β注射到大鼠的踝关节诱导急性骨关节炎模型,注射后24 h检测关节灌洗液以及整个关节组织的基因表达和组织病理学。100 ng的白细胞介素1β即可引起关节促炎和分解代谢介质的基因表达上调,包括白细胞介素6、前列腺素内过氧化物合成酶2(PTGS2)、诱导型一氧化氮合酶(NOS2)、肿瘤坏死因子α、核因子κB、ADAMTS5和白细胞介素1β;关节灌洗液的生化分析显示氨基聚糖(glycosaminoglycan,GAG)、白细胞介素6蛋白和NO的积累;造模24 h,组织病理学已显示确切的滑膜炎改变,但因造模时间尚短,没有软骨破坏的组织学证据,但氨基多糖释放到关节灌洗液中可能表明早期细胞外基质降解。该模型的优势包括在4 h内诱导可重复的关节炎症和氨基多糖释放,有助于白细胞介素1通路拮抗剂的初始评估,但其效用受到研究持续时间短和疾病启动的非生理学方法的限制。 (2)大鼠踝关节软骨萎缩骨关节炎模型:此方法是通过关节制动,限制关节活动使关节周围软组织挛缩,从而改变关节周围应力,导致软骨萎缩、软骨下骨异常骨改建而诱导骨关节炎的方法。VASILCEAC等[22]采用Wistar大鼠踝关节固定化的方法,分析了大鼠踝关节胶原纤维的总密度、粗胶原纤维的密度和细胶原纤维的密度,比较了不同组的左右后肢,结果显示,固定化促进了细纤维和总胶原蛋白密度的降低;固定后的关节胶原纤维丢失,固定后的肌肉锻炼方案只能恢复细的胶原纤维。该模型可能有助于研究预防踝关节固定后软骨萎缩,并且与慢性踝关节不稳模型相结合,可以深入了解关节固定在慢性踝关节不稳的退行性和愈合过程中的重要性。 (3)大鼠踝关节衰老骨关节炎模型:增龄大鼠模型中可研究自发性骨关节炎的疾病过程。通过使用增龄动物,创造骨关节炎衰老模型。MORIYAMA等[23]描述了26个月大的CD/BR Sprague Dawley大鼠的自发性踝关节骨关节炎,病变范围主要集中在软骨组织及软骨下骨,滑膜病变出现较少,包括局灶性软骨细胞坏死、蛋白多糖丢失、软骨丢失以及软骨层钙化的全层病变,组织学切片表明局部增加的接触压力可能是引起踝骨关节炎的诱因;另外,雄性大鼠发病率高于雌性,这可能与雄性较高的体质量和内分泌因素引起的机械因素有关。这项研究表明,与该模型中的髋关节和膝关节相比,踝关节的骨关节炎变化的发生率和严重程度最高。但在人类中,膝关节与退行性软骨改变始终比同一个体的踝关节更严重。 (4) STR/ORT小鼠自发性踝骨关节炎模型:STR/ORT小鼠是自发性膝关节骨关节炎的成熟模型[24],雄性易受累,关节周围软组织结构的早期钙化是该模型的突出特征[25]。STR/ORT小鼠膝关节和踝关节骨关节炎的进展不同;在雄性小鼠中,膝关节骨关节炎与年龄的增长相关,而踝关节骨关节炎在五六月龄的小鼠中发病率显著增加,后趋于平稳,组织学显示,踝关节周围异常增生骨质形成,距骨骨间韧带从3月龄左右开始矿化;膝关节和踝关节骨关节炎的发展在单个小鼠内是独立的。STR/ORT小鼠骨关节炎的病因尚不完全清楚,但目前基于转录谱的Meta分析显示,软骨内骨化相关的基因表达被上调,基质金属蛋白酶13和X型胶原表达增加,组织矿化调节因子的差异表达,表明与软骨内生长相关的固有软骨细胞存在缺陷[26]。在该模型中,在软骨变性前的软组织钙化过多,病理生理学不能反映人类踝关节骨关节炎。 (5) BCBC/Y 小鼠踝关节发育不良骨关节炎模型:BCBC/Y小鼠是从一种肉桂色毛发、骨关节病易感的B6C3F1小鼠中诞生,这种小鼠的特征是脚尖走路,骨关节炎改变,如骨组织的侵蚀或融合,在其踝关节中尤为明显。在BCBC/Y小鼠低龄期阶段,组织病理学上即观察到软骨退行性改变,进而可观察到关节软骨松动、裂缝和侵蚀,其特征在于与异常骨赘的关节融合;从9月龄左右开始出现进行性踝关节肿胀;到10-20个月时,这些小鼠表现出异常的姿势和步态。在组织学检查中,BCBC/Y小鼠早期软骨病变包括软骨细胞坏死、软骨纤维化和变薄,进而发展至包括全层软骨缺损以及软骨肥大化,严重的骨赘进展为关节强直,最后导致踝关节的关节融合[27]。这种非典型关节病理变化存在的疾病机制和潜在的遗传因素,对踝关节的偏好尚不能确定。 (6)豚鼠自发性踝骨关节炎模型:Dunkin-Hartley豚鼠作为骨关节炎常用动物模型,自发性骨关节炎相对容易成模,不需通过人为干预,仅令其自由活动,即可成模。此模型与人类原发性骨关节炎发病较相近,可通过此模型研究人类原发性骨关节炎的病理生理过程及环境[28]。通过组织学染色,可反映骨关节炎的特异性表征,在3月龄时,Dunkin-Hartley豚鼠踝关节出现滑膜炎表征,一部分豚鼠踝关节出现局限性软骨面磨损及轻度软骨退行性改变;6月龄时,踝关节均有中度局限性软骨退行性变、软骨细胞外基质丢失、软骨细胞凋亡,并不同程度的出现了边缘性骨赘增生和软骨下骨改变;并且随着年龄的增长Caspase3表达显著增加,软骨裂缝加深,范围变大,软骨细胞肥大化相关蛋白的表达增加,如X型胶原及基质金属蛋白酶13。 各类踝关节骨关节炎动物模型特点总结,见表2。"
| [1] MURRAY C, MARSHALL M, RATHOD T, et al. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: A systematic review and cross-sectional study. PLoS One. 2018;13(4):e193662. [2] KRAMER WC, HENDRICKS KJ, WANG J, et al. Pathogenetic mechanisms of posttraumatic osteoarthritis: opportunities for early intervention. Int J Clin Exp Med. 2011;4(4):285-298. [3] BARG A, PAGENSTERT GI, HÜGLE T, et al. Ankle osteoarthritis: etiology, diagnostics, and classification. Foot Ankle Clin. 2013;18(3):411-426. [4] AICHER WK, ROLAUFFS B. The spatial organisation of joint surface chondrocytes: review of its potential roles in tissue functioning, disease and early, preclinical diagnosis of osteoarthritis. Ann Rheum Dis. 2014; 73(4):645-653. [5] BERNASCONI A, COOPER L, LYLE S, et al. Intraobserver and interobserver reliability of cone beam weightbearing semi-automatic three-dimensional measurements in symptomatic pes cavovarus. Foot Ankle Surg. 2020;26(5):564-572. [6] GOLDITZ T, STEIB S, PFEIFER K, et al. Functional ankle instability as a risk factor for osteoarthritis: using T2-mapping to analyze early cartilage degeneration in the ankle joint of young athletes. Osteoarthritis Cartilage. 2014;22(10):1377-1385. [7] VALDERRABANO V, HORISBERGER M, RUSSELL I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467(7):1800-1806. [8] LUBBEKE A, SALVO D, STERN R, et al. Risk factors for post-traumatic osteoarthritis of the ankle: an eighteen years follow-up study. Int Orthop. 2012;36(7):1403-1410. [9] STUFKENS SA, KNUPP M, HORISBERGER M, et al. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286. [10] KHLOPAS H, KHLOPAS A, SAMUEL LT, et al. Current Concepts in Osteoarthritis of the Ankle: Review. Surg Technol Int. 2019;35:280-294. [11] THOMAS AC, HUBBARD-TURNER T, WIKSTROM EA, et al. Epidemiology of Posttraumatic Osteoarthritis. J Athl Train. 2017;52(6):491-496. [12] DAHMEN J, JADDI S, HAGEMEIJER NC, et al. Incidence of (Osteo)Chondral Lesions of the Ankle in Isolated Syndesmotic Injuries: A Systematic Review and Meta-Analysis. Cartilage. 2022;13(2): 788750359. [13] GATLIN CC, MATHENY LM, HO CP, et al. Diagnostic accuracy of 3.0 Tesla magnetic resonance imaging for the detection of articular cartilage lesions of the talus. Foot Ankle Int. 2015;36(3):288-292. [14] JALEEL A, GOLIGHTLY YM, ALVAREZ C, et al. Incidence and progression of ankle osteoarthritis: The johnston county osteoarthritis project. Semin Arthritis Rheum. 2021;51(1):230-235. [15] LI J, CHEN Z, CHENG Y, et al. Ligamentous injury-induced ankle instability causing posttraumatic osteoarthritis in a mouse model. BMC Musculoskelet Disord. 2022;23(1):223. [16] TSAI CH, CHEN CJ, GONG CL, et al. CXCL13/CXCR5 axis facilitates endothelial progenitor cell homing and angiogenesis during rheumatoid arthritis progression. Cell Death Dis. 2021;12(9):846. [17] CHANG S H, YASUI T, TAKETOMI S, et al. Comparison of mouse and human ankles and establishment of mouse ankle osteoarthritis models by surgically-induced instability. Osteoarthritis Cartilage. 2016; 24(4):688-697. [18] ZHAO X, KIM D, SUMINDA G, et al. Inhibitory Effects of IL-6-Mediated Matrix Metalloproteinase-3 and -13 by Achyranthes japonica Nakai Root in Osteoarthritis and Rheumatoid Arthritis Mice Models. Pharmaceuticals (Basel). 2021;14(8):776. [19] BIELAJEW BJ, HU JC, ATHANASIOU KA. Methodology to Quantify Collagen Subtypes and Crosslinks: Application in Minipig Cartilages. Cartilage. 2021;13(2_suppl):1742S-1754S. [20] GOETZ J E, FREDERICKS D, PETERSEN E, et al. A clinically realistic large animal model of intra-articular fracture that progresses to post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(10): 1797-1805. [21] SCOTT I, MIDHA A, RASHID U, et al. Correlation of gene and mediator expression with clinical endpoints in an acute interleukin-1beta-driven model of joint pathology. Osteoarthritis Cartilage. 2009;17(6):790-797. [22] VASILCEAC FA, RENNER AF, TEODORO WR, et al. The remodeling of collagen fibers in rats ankles submitted to immobilization and muscle stretch protocol. Rheumatol Int. 2011;31(6):737-742. [23] MORIYAMA H, KANEMURA N, BROUNS I, et al. Effects of aging and exercise training on the histological and mechanical properties of articular structures in knee joints of male rat. Biogerontology. 2012; 13(4):369-381. [24] KANAKIS I, LIU K, POULET B, et al. Targeted Inhibition of Aggrecanases Prevents Articular Cartilage Degradation and Augments Bone Mass in the STR/Ort Mouse Model of Spontaneous Osteoarthritis. Arthritis Rheumatol. 2019;71(4):571-582. [25] WANG G, CHEN S, XIE Z, et al. TGFβ attenuates cartilage extracellular matrix degradation via enhancing FBXO6-mediated MMP14 ubiquitination. Ann Rheum Dis. 2020;79(8):1111-1120. [26] STAINES KA, MADI K, MIRCZUK SM, et al. Endochondral Growth Defect and Deployment of Transient Chondrocyte Behaviors Underlie Osteoarthritis Onset in a Natural Murine Model. Arthritis Rheumatol. 2016;68(4):880-891. [27] THOMSEN JS, STRAARUP TS, DANIELSEN CC, et al. Relationship between articular cartilage damage and subchondral bone properties and meniscal ossification in the Dunkin Hartley guinea pig model of osteoarthritis. Scand J Rheumatol. 2011;40(5):391-399. [28] MINTON DM, ELLIEHAUSEN CJ, JAVORS MA, et al. Rapamycin-induced hyperglycemia is associated with exacerbated age-related osteoarthritis. Arthritis Res Ther. 2021;23(1):253. [29] KHELLA C M, ASGARIAN R, HORVATH J M, et al. An Evidence-Based Systematic Review of Human Knee Post-Traumatic Osteoarthritis (PTOA): Timeline of Clinical Presentation and Disease Markers, Comparison of Knee Joint PTOA Models and Early Disease Implications. Int J Mol Sci. 2021;22(4):1996. [30] LAI-ZHAO Y, PITCHERS KK, APPLETON CT. Transient anabolic effects of synovium in early post-traumatic osteoarthritis: a novel ex vivo joint tissue co-culture system for investigating synovium-chondrocyte interactions. Osteoarthritis Cartilage. 2021;29(7):1060-1070. [31] PASTOUREAU PC, HUNZIKER EB, PELLETIER JP. Cartilage, bone and synovial histomorphometry in animal models of osteoarthritis. Osteoarthritis Cartilage. 2010;18 Suppl 3:S106-S112. [32] VERONESI F, FINI M, MARTINI L, et al. In Vivo Model of Osteoarthritis to Compare Allogenic Amniotic Epithelial Stem Cells and Autologous Adipose Derived Cells. Biology (Basel). 2022;11(5):681. [33] MCCREADY E, EASLEY JT, RISCH M, et al. Biomechanical, Morphological, and Biochemical Characteristics of Articular Cartilage of the Ovine Humeral Head. Cartilage. 2022;13(1):788771463. [34] ORTH P, ELDRACHER M, CUCCHIARINI M, et al. Small-Diameter Subchondral Drilling Improves DNA and Proteoglycan Content of the Cartilaginous Repair Tissue in a Large Animal Model of a Full-Thickness Chondral Defect. J Clin Med. 2020;9(6):1903. [35] MCILWRAITH CW, FRISBIE DD, KAWCAK CE. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. 2012;1(11):297-309. [36] BARKER WH, SMITH MR, MINSHALL GJ, et al. Soft tissue injuries of the tarsocrural joint: a retrospective analysis of 30 cases evaluated arthroscopically. Equine Vet J. 2013;45(4):435-441. [37] LEE MI, KIM JH, KWAK HH, et al. A placebo-controlled study comparing the efficacy of intra-articular injections of hyaluronic acid and a novel hyaluronic acid-platelet-rich plasma conjugate in a canine model of osteoarthritis. J Orthop Surg Res. 2019;14(1):314. [38] KRISTON-PÁL É, HARACSKA L, COOPER P, et al. A Regenerative Approach to Canine Osteoarthritis Using Allogeneic, Adipose-Derived Mesenchymal Stem Cells. Safety Results of a Long-Term Follow-Up. Front Vet Sci. 2020;7:510. [39] BERAUD R, MOREAU M, LUSSIER B. Effect of exercise on kinetic gait analysis of dogs afflicted by osteoarthritis. Vet Comp Orthop Traumatol. 2010;23(2):87-92. [40] LAMPROPOULOU-ADAMIDOU K, LELOVAS P, KARADIMAS E V, et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2014;24(3):263-271. [41] PASCUAL GC, HAKIMIYAN AA, RAPPOPORT L, et al. Anti-apoptotic treatments prevent cartilage degradation after acute trauma to human ankle cartilage. Osteoarthritis Cartilage. 2009;17(9):1244-1251. [42] TOCHIGI Y, BUCKWALTER JA, MARTIN JA, et al. Distribution and progression of chondrocyte damage in a whole-organ model of human ankle intra-articular fracture. J Bone Joint Surg Am. 2011;93(6): 533-539. [43] BAJAJ S, SHOEMAKER T, HAKIMIYAN AA, et al. Protective effect of P188 in the model of acute trauma to human ankle cartilage: the mechanism of action. J Orthop Trauma. 2010;24(9):571-576. [44] SHANG X, FANG Y, XIN W, et al. The Application of Extracellular Vesicles Mediated miRNAs in Osteoarthritis: Current Knowledge and Perspective. J Inflamm Res. 2022;15:2583-2599. [45] KOKKOTIS C, MOUSTAKIDIS S, BALTZOPOULOS V, et al. Identifying Robust Risk Factors for Knee Osteoarthritis Progression: An Evolutionary Machine Learning Approach. Healthcare (Basel). 2021;9(3):260. [46] GORBACHOVA T, WANG PS, HU B, et al. Plantar talar head contusions and osteochondral fractures: associated findings on ankle MRI and proposed mechanism of injury. Skeletal Radiol. 2016;45(6):795-803. [47] TAO H, HU Y, QIAO Y, et al. T(2) -Mapping evaluation of early cartilage alteration of talus for chronic lateral ankle instability with isolated anterior talofibular ligament tear or combined with calcaneofibular ligament tear. J Magn Reson Imaging. 2018;47(1):69-77. [48] MÜLLER-FRANZES G, NOLTE T, CIBA M, et al. Fast, Accurate, and Robust T2 Mapping of Articular Cartilage by Neural Networks. Diagnostics (Basel). 2022;12(3):688. [49] ÄNGEBY MK, AULIN C, BAHARPOOR A, et al. Pain behaviour assessments by gait and weight bearing in surgically induced osteoarthritis and inflammatory arthritis. Physiol Behav. 2020;225:113079. |
| [1] | Nong Fuxiang, Jiang Zhixiong, Li Yinghao, Xu Wencong, Shi Zhilan, Luo Hui, Zhang Qinglang, Zhong Shuang, Tang Meiwen. Bone cement augmented proximal femoral nail antirotation for type A3.3 intertrochanteric femoral fracturalysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-10. |
| [2] | Li Xiaomin, Tian Xiangdong, Tan Yetong, Zhu Guangyu, Wang Rongtian, Wang Jian, Xue Zhipeng, Ma Sheng, Hu Yuanyi, Huang Ye, Ding Tiansong. Changes of lower limb force line and knee function after high tibial osteotomy in osteoporotic medial ventricular knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1325-1329. |
| [3] | Pan Zhongjie, Qin Zhihong, Zheng Tiejun, Ding Xiaofei, Liao Shijie. Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1441-1447. |
| [4] | Cai Zhihao, Xie Zhaoyong. Femoral neck anteversion measurement assessment: how to establish a unified method and standard [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1448-1454. |
| [5] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [6] | Huang Linke, Wei Linhua, Jiang Jie, Liu Qian, Chen Weiwei. Effects of estrogen combined with treadmill exercise on bone mass and articular cartilage in ovariectomized mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1166-1171. |
| [7] | Ruan Ling, Wang Guanghua, Wu Rongping, Jin Zhan, Lyu Zhenqing, Zhang Nan, Li Shoubang. Correlation between exercise intensity and lipid metabolism disorder and oxidative stress in a high-diet rat model [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1149-1155. |
| [8] | Wang Ji, Zhang Min, Yang Zhongya, Zhang Long. A review of physical activity intervention in type 2 diabetes mellitus with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1272-1277. |
| [9] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [10] | Gao Yu, Han Jiahui, Ge Xin. Immunoinflammatory microenvironment after spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1300-1305. |
| [11] | Xu Xingxing, Wen Chaoju, Meng Maohua, Wang Qinying, Chen Jingqiao, Dong Qiang. Carbon nanomaterials in oral implant [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1062-1070. |
| [12] | Li Cheng, Zheng Guoshuang, Kuai Xiandong, Yu Weiting. Alginate scaffold in articular cartilage repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1080-1088. |
| [13] | Chen Shisong, Liu Xiaohong, Xu Zhiyun. Current status and prospects of bioprosthetic heart valves [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1096-1102. |
| [14] | Shi Yehong, Wang Cheng, Chen Shijiu. Early thrombosis and prevention of small-diameter blood vessel prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1110-1116. |
| [15] | Tang Haotian, Liao Rongdong, Tian Jing. Application and design of piezoelectric materials for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1117-1125. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||