Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (16): 2525-2533.doi: 10.12307/2023.463
Previous Articles Next Articles
Antibacterial and hemostatic properties of chitosan collagen sponge
Zhu Hong, Lin Ziheng, He Rouye, Pan Jinbin, Liu Xiaochuan, He Xiaoling, Zhang Jingying
- Guangdong Medical University, First Affiliated Hospital of Guangdong Medical University, Key Laboratory of 3D Printing of Stomatology, Dongguan 523710, Guangdong Province, China
-
Received:2022-06-25Accepted:2022-07-29Online:2023-06-08Published:2022-11-10 -
Contact:Zhang Jingying, PhD, Associate professor, Guangdong Medical University, First Affiliated Hospital of Guangdong Medical University, Key Laboratory of 3D Printing of Stomatology, Dongguan 523710, Guangdong Province, China -
About author:Zhu Hong, Master candidate, Guangdong Medical University, First Affiliated Hospital of Guangdong Medical University, Key Laboratory of 3D Printing of Stomatology, Dongguan 523710, Guangdong Province, China -
Supported by:the Project of Innovation Experiment for College Students of Guangdong Medical University, No. FCZF002 (to ZH); Project Establishment of Innovation and Entrepreneurship Training Program for College Students in Guangdong Province, No. GDMU2021106 (to HRY); the Basic and Applied Basic Research of Guangdong Province, No. 2020B1515120001 (to ZJY); Key Fields Foundation of Colleges and Universities in Guangdong Province, No. 2020ZDZX2013 (to ZJY); Discipline Construction Project of Guandong Medical University, No. 4SG21015G, No. 4SG21019G (to ZJY)
CLC Number:
Cite this article
Zhu Hong, Lin Ziheng, He Rouye, Pan Jinbin, Liu Xiaochuan, He Xiaoling, Zhang Jingying. Antibacterial and hemostatic properties of chitosan collagen sponge[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2525-2533.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
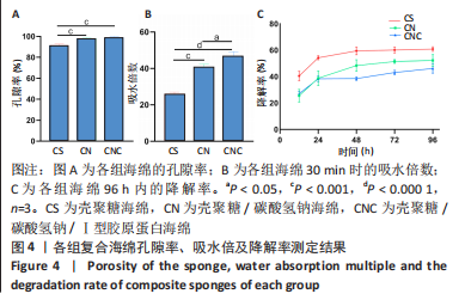
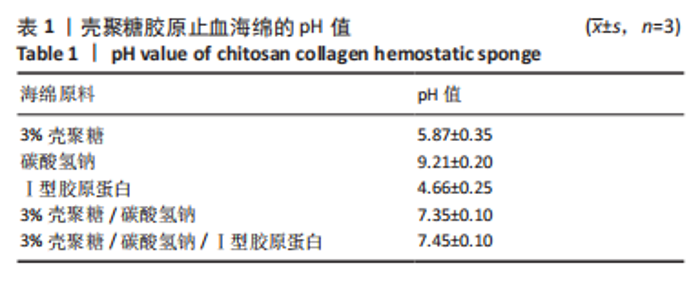
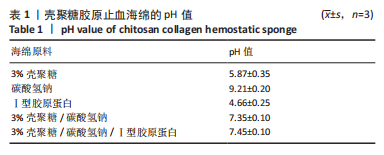
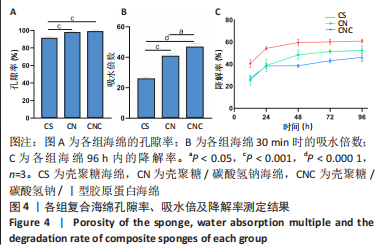
2.4 海绵降解曲线、孔隙率及吸水倍数 图4A显示壳聚糖/碳酸氢钠/Ⅰ型胶原海绵孔隙率最大,孔隙率平均为99.13%,大于壳聚糖海绵的平均孔隙率91.57% (P < 0.01)及壳聚糖/碳酸氢钠海绵的平均孔隙率98.12% (P < 0.01)。图4B显示了海绵30 min内的吸水倍数,经测量显示,壳聚糖海绵30 min内的平均吸水倍数为25.88倍,而碳酸氢钠加入后的壳聚糖/碳酸氢钠海绵和壳聚糖/碳酸氢钠/Ⅰ型胶原海绵吸水效果明显增加,平均吸水倍数分别为40.75倍(P < 0.000 1)和46.74倍(P < 0.05)。降解实验表明壳聚糖/碳酸氢钠/Ⅰ型胶原海绵的降解速率最慢,96 h时仅降解46.15%;壳聚糖/碳酸氢钠海绵降解率次之,96 h时降解52.20%;而壳聚糖海绵降解速率最快,96 h时降解60.86%,见图4C。"
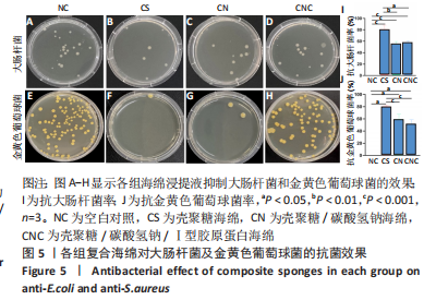
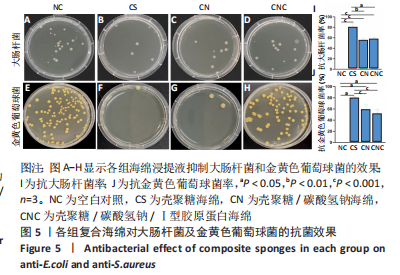
2.5 海绵抗菌效果 图5A-H表明,壳聚糖海绵的抑菌效果最佳,抗大肠杆菌率平均为80.58%,抗金黄色葡萄球菌率平均为80.14%;碳酸氢钠的加入在改变pH值的同时降低抗菌率,壳聚糖/碳酸氢钠海绵抗大肠杆菌率平均为55.06%,抗金黄色葡萄球菌率平均为59.06%;壳聚糖/碳酸氢钠/Ⅰ型胶原海绵的抗大肠杆菌率平均为57.56%,抗金黄色葡萄球菌率平均为51.21%,综上所述,抗大肠杆菌率:壳聚糖>壳聚糖/碳酸氢钠海绵>壳聚糖/碳酸氢钠/Ⅰ型胶原蛋白海绵(P < 0.001,P < 0.000 1),抗金黄色葡萄球菌率:壳聚糖海绵>壳聚糖/碳酸氢钠海绵>壳聚糖/碳酸氢钠/Ⅰ型胶原蛋白海绵(P < 0.01),见图5I,J。"
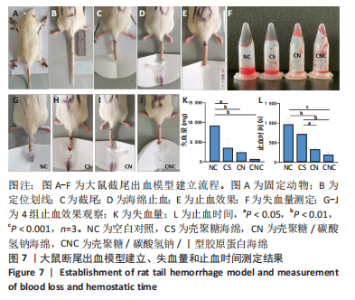
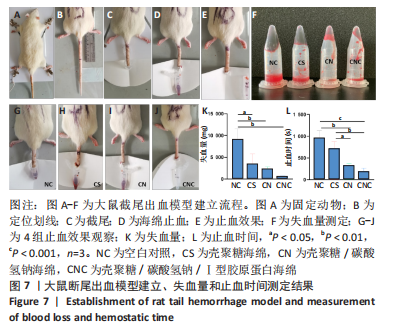
2.7 海绵止血效果 采用大鼠断尾出血模型评价海绵的止血能力,见图7A-F。选用12只大鼠进行大鼠断尾出血实验,结果显示,空白对照组自然完全凝血时间平均为963.33 s(P < 0.05),失血量平均为9 153.67 mg;覆盖壳聚糖海绵的鼠尾止血时间平均为718 s(P < 0.01),失血量平均为3 419.37 mg(P < 0.05);覆盖壳聚糖/碳酸氢钠海绵的鼠尾止血时间平均为321 s(P < 0.05),失血量平均为2 270.84 mg(P < 0.001);而壳聚糖/碳酸氢钠/Ⅰ型胶原海绵在178 s(P < 0.01)即可使尾部血液凝固,失血量平均为553.83 mg,见图7K-L,壳聚糖/碳酸氢钠/Ⅰ型胶原海绵止血效果优于壳聚糖海绵及壳聚糖/碳酸氢钠海绵。"

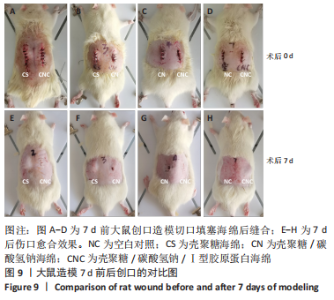
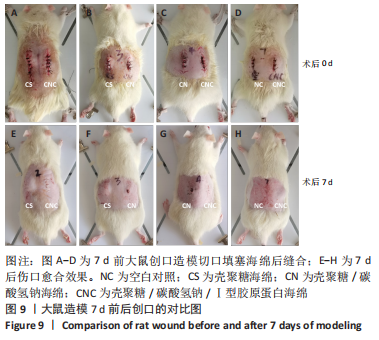
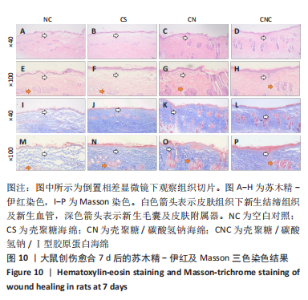
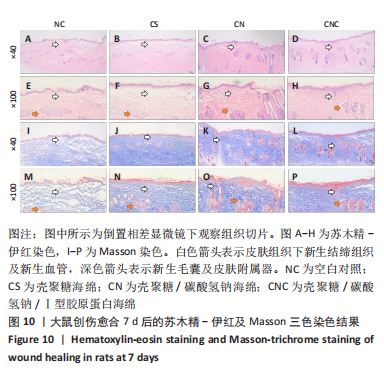
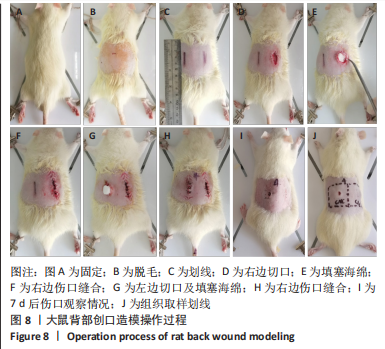
2.8 海绵促创口愈合效果 采用大鼠皮肤创伤模型评价海绵的促组织愈合效果,模型建立流程见图8A-J。选用12只大鼠进行皮肤创伤实验,分别填塞3种不同成分的海绵,见图9。大鼠创口造模7 d后,壳聚糖/碳酸氢钠海绵填塞的大鼠背部伤口仍有结痂,大鼠背部隆起,海绵大部分未降解,而壳聚糖/碳酸氢钠/Ⅰ型胶原海绵填塞的伤口无结痂,大部分被降解。图10显示将大鼠伤口处的皮肤及皮下组织进行苏木精-伊红染色、Masson三色染色及苏木精-伊红染色显示,术后7 d,空白对照组的皮肤结缔组织出现不同程度的断裂,炎症细胞增多,见图10A,I。壳聚糖海绵组及壳聚糖/碳酸氢钠海绵相较于空白对照组而言,无明显炎症反应且毛细血管生成明显增多,成纤维细胞明显增生且有序,见图10B,C,J,K,壳聚糖/碳酸氢钠/Ⅰ型胶原海绵覆盖填塞的组织血管及纤维组织增生最多且致密,皮肤角质层明显增厚,皮下组织中可见众多毛囊样结构,皮肤结构完整且有序,见图10D,L。该实验表明:三组海绵均可有效促进皮肤创口的愈合,其中壳聚糖/碳酸氢钠/Ⅰ型胶原海绵效果最佳,更能恢复皮肤及皮下组织的正常组织结构。"
| [1] CHENG H, SHI Z, YUE K, et al. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021;124:219-232. [2] KUMBARGERE NAGRAJ S, PRASHANTI E, AGGARWAL H, et al. Interventions for treating post-extraction bleeding. Cochrane Database Syst Rev. 2018;3(3):Cd011930. [3] 王静娟,胡开进,刘平,等.拔牙窝止血类覆盖及充填材料的选择及应用[J].中国实用口腔科杂志,2017,10(10):586-589. [4] YOSHIDA K, KODAMA Y, NAGAI T, et al. Clinico-statistical survey of oral antimicrobial prophylaxis and surgical site infection regarding ordinary tooth extraction and mandibular wisdom tooth extraction in the dental outpatient clinic. J Infect Chemother. 2021;27(2):192-197. [5] HEMMINGSEN LM, JULIN K, AHSAN L, et al. Chitosomes-in-chitosan hydrogel for acute skin injuries: prevention and infection control. Mar Drugs. 2021;19(5):269. [6] AGUILAR A, ZEIN N, HARMOUCH E, et al. Application of chitosan in bone and dental engineering. Molecules. 2019;24(16):3009. [7] NARVAEZ-FLORES JJ, VILAR-PINEDA G, ACOSTA-TORRES LS, et al. Cytotoxic and anti-inflammatory effects of chitosan and hemostatic gelatin in oral cell culture. Acta Odontol Latinoam. 2021;34(2):98-103. [8] KOU SG, PETERS LM, MUCALO MR. Chitosan: a review of sources and preparation methods. Int J Biol Macromol. 2021;169:85-94. [9] ABDELALLAH NH, GABER Y, ABDELGHANI S, et al. Chitosan and alginate salt as biomaterials are potential natural adjuvants for killed cholera vaccine. J Biomed Mater Res A. 2021;109(12):2462-2470. [10] GAO X, LIU N, WANG Z, et al. Development and optimization of chitosan nanoparticle-based intranasal vaccine carrier. Molecules. 2021;27(1):204. [11] CAO S, YANG Y, ZHANG S, et al. Multifunctional dopamine modification of green antibacterial hemostatic sponge. Mater Sci Eng C Mater Biol Appl. 2021;127:112227. [12] HUANG Y, FENG L, ZHANG Y, et al. Hemostasis mechanism and applications of N-alkylated chitosan sponge. Polym Adv Tech. 2017; 28(9):1107-1114. [13] VERLEE A, MINCKE S, STEVENS CV. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr Polym. 2017;164:268-283. [14] ISLAM S, ARNOLD L, PADHYE R. Comparison and characterisation of regenerated chitosan from 1-butyl-3-methylimidazolium chloride and chitosan from crab shells. Biomed Res Int. 2015;2015:874316. [15] ABD EL-HACK ME, EL-SAADONY MT, SHAFI ME, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int J Biol Macromol. 2020;164:2726-2744. [16] WANG W, MENG Q, LI Q, et al. Chitosan derivatives and their application in biomedicine. Int J Mol Sci. 2020;21(2):487. [17] HALL CW, MAH TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41(3):276-301. [18] MALETTE WG, QUIGLEY HJ, GAINES RD, et al. Chitosan: a new hemostatic. Ann Thorac Surg. 1983;36(1):55-58. [19] KONG M, CHEN XG, XING K, et al. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51-63. [20] LU B, WANG T, LI Z, et al. Healing of skin wounds with a chitosan-gelatin sponge loaded with tannins and platelet-rich plasma. Int J Biol Macromol. 2016;82:884-891. [21] 刘海燕,胡阳,武秀萍,等.壳聚糖基多糖材料防治口腔疾病的作用[J].中国组织工程研究,2020,24(4):631-636. [22] CHOWDHURY SR, MH BUSRA MF, LOKANATHAN Y, et al. Collagen type I: a versatile biomaterial. Adv Exp Med Biol. 2018;1077:389-414. [23] FERREIRA AM, GENTILE P, CHIONO V, et al. Collagen for bone tissue regeneration. Acta Biomater. 2012;8(9):3191-3200. [24] RITTIé L. Type I collagen purification from rat tail tendons. Methods Mol Biol. 2017;1627:287-308. [25] NEVE A, CANTATORE FP, MARUOTTI N, et al. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int. 2014;2014:756078. [26] SONG WK, LIU D, SUN LL, et al. Physicochemical and biocompatibility properties of type I collagen from the skin of nile tilapia (oreochromis niloticus) for biomedical applications. Mar Drugs. 2019;17(3):137. [27] ZHU Y, LIU W, CHEN S, et al. Collagen type I enhances cell growth and insulin biosynthesis in rat pancreatic cells. J Mol Endocrinol. 2021; 67(3):135-148. [28] LUO J, MENG Y, ZHENG L, et al. Fabrication and characterization of Chinese giant salamander skin composite collagen sponge as a high-strength rapid hemostatic material. J Biomater Sci Polym Ed. 2018; 29(16):1933-1948. [29] 谢海霞,沈先荣,葛卫红,等.胶原蛋白-壳聚糖-海藻酸盐复合敷料的促愈合作用. 药物生物技术,2016,23(6):495-502. [30] GHODBANE SA, DUNN MG. Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. J Biomed Mater Res A. 2016;104(11):2685-2692. [31] ALVEN S, ADERIBIGBE BA. Chitosan and cellulose-based hydrogels for wound management. Int J Mol Sci. 2020;21(24):9656. [32] GAUTAM S, CHOU CF, DINDA AK, et al. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;34:402-409. [33] CHEN X, ZHANG M, WANG X, et al. Peptide-modified chitosan hydrogels promote skin wound healing by enhancing wound angiogenesis and inhibiting inflammation. Am J Transl Res. 2017;9(5):2352-2362. [34] LI J, WANG H, ZHOU H, et al. Fabrication of highly interconnected poly(ε-caprolactone)/cellulose nanofiber composite foams by microcellular foaming and leaching processes. ACS Omega. 2021;6(35): 22672-22680. [35] JIANG X, WANG Y, FAN D, et al. A novel human-like collagen hemostatic sponge with uniform morphology, good biodegradability and biocompatibility. J Biomater Appl. 2017;31(8):1099-1107. [36] MATICA MA, AACHMANN FL, TøNDERVIK A, et al. Chitosan as a wound dressing starting material: antimicrobial properties and mode of action. Int J Mol Sci. 2019;20(23):5889. [37] HU Z, ZHANG DY, LU ST, et al. Chitosan-based composite materials for prospective hemostatic applications. Mar Drugs. 2018;16(8):273. [38] MOUSAVI S, KHOSHFETRAT AB, KHATAMI N, et al. Comparative study of collagen and gelatin in chitosan-based hydrogels for effective wound dressing: physical properties and fibroblastic cell behavior. Biochem Biophys Res Commun. 2019;518(4):625-631. [39] GEDAR TOTUK ÖM, GüZEL ŞE, EKICI H, et al. Effects of Algan Hemostatic Agent on bleeding time in a rat tail hemorrhage model. Ulus Travma Acil Cerrahi Derg. 2020;26(6):853-858. [40] YUAN J, HOU Q, CHEN D, et al. Chitosan/LiCl composite scaffolds promote skin regeneration in full-thickness loss. Sci China Life Sci. 2020;63(4):552-562. [41] ARWEILER NB, NETUSCHIL L. The oral microbiota. Adv Exp Med Biol. 2016;902:45-60. [42] WANG X, GUO J, ZHANG Q, et al. Gelatin sponge functionalized with gold/silver clusters for antibacterial application. Nanotechnology. 2020;31(13):134004. [43] HWANG SJ, HA GH, SEO WY, et al. Human collagen alpha-2 type I stimulates collagen synthesis, wound healing, and elastin production in normal human dermal fibroblasts (HDFs). BMB Rep. 2020;53(10): 539-544. [44] ZHANG W, ZHAO L, GAO C, et al. Highly resilient, biocompatible, and antibacterial carbon nanotube/hydroxybutyl chitosan sponge dressing for rapid and effective hemostasis. J Mater Chem B. 2021;9(47): 9754-9763. [45] FAN X, LI M, YANG Q, et al. Morphology-controllable cellulose/chitosan sponge for deep wound hemostasis with surfactant and pore-foaming agent. Mater Sci Eng C Mater Biol Appl. 2021;118:111408. [46] GADKARI RR, SUWALKA S, YOGI MR, et al. Green synthesis of chitosan-cinnamaldehyde cross-linked nanoparticles: characterization and antibacterial activity. Carbohydr Polym. 2019;226:115298. [47] RICO-LLANOS GA, BORREGO-GONZáLEZ S, MONCAYO-DONOSO M, et al. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers (Basel). 2021;13(4):599. [48] SEIDI F, KHODADADI YAZDI M, JOUYANDEH M, et al. Chitosan-based blends for biomedical applications. Int J Biol Macromol. 2021;183: 1818-1850. [49] 汤宋佳.壳聚糖—胶原海绵敷料的制备及其对伤口愈合的作用研究[D].杭州:浙江大学,2016. [50] BARRS RW, JIA J, SILVER SE, et al. Biomaterials for bioprinting microvasculature. Chem Rev. 2020;120(19):10887-10949. [51] 柯林楠,冯晓明,王春仁.医用敷料研究的现状与进展[J].中国组织工程研究与临床康复,2010,14(3):521-524. [52] JAYAKUMAR R, PRABAHARAN M, SUDHEESH KUMAR PT, et al. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322-337. [53] VARMA S, ORGEL JP, SCHIEBER JD. Nanomechanics of type I collagen. Biophys J. 2016;111(1):50-56. [54] CHATTOPADHYAY S, RAINES RT. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821-833. [55] SHARIATINIA Z. Carboxymethyl chitosan: properties and biomedical applications. Int J Biol Macromol. 2018;120(Pt B):1406-1419. [56] SAHANA TG, REKHA PD. Biopolymers: applications in wound healing and skin tissue engineering. Mol Biol Rep. 2018;45(6):2857-2867. [57] SHI C, ZHU Y, RAN X, et al. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192. [58] ZHANG MX, ZHAO WY, FANG QQ, et al. Effects of chitosan-collagen dressing on wound healing in vitro and in vivo assays. J Appl Biomater Funct Mater. 2021;19:2280800021989698. [59] LIU L, TANG X, WANG Y, et al. Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system. Int J Pharm. 2011;414(1-2):6-15. [60] GUYOT C, CERRUTI M, LEROUGE S. Injectable, strong and bioadhesive catechol-chitosan hydrogels physically crosslinked using sodium bicarbonate. Mater Sci Eng C Mater Biol Appl. 2021;118:111529. |
| [1] | Lian Shilin, Zhang Yan, Jiang Qiang, Zhang Hanshuo, Li Tusheng, Ding Yu. Interventional effects of whole blood and platelet-rich plasma with different preparation methods on nucleus pulposus cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1199-1204. |
| [2] | Xu Xingxing, Wen Chaoju, Meng Maohua, Wang Qinying, Chen Jingqiao, Dong Qiang. Carbon nanomaterials in oral implant [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1062-1070. |
| [3] | Li Rui, Liu Zhen, Guo Zige, Lu Ruijie, Wang Chen. Aspirin-loaded chitosan nanoparticles and polydopamine modified titanium sheets improve osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 374-379. |
| [4] | Li Yue, Lyu Yan, Feng Wanying, Song Yang, Yan Yu, Guan Yongge. Preparation of hyperoside nanoparticles to repair endometrial injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 360-366. |
| [5] | Wu Lihao, Shao Anliang, Xu Lin, Ren Kang, Wang Hongjian, Chen Liang, Xu Ling. Evaluation of immunotoxicity of the absorbable macroporous polysaccharides composite hemostatic material [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 329-334. |
| [6] | Zhou Jie, Pei Xibo, Wan Qianbing. Advances and biological application of asymmetric dressings [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 434-440. |
| [7] | Zong Mingrui, Liu Haiyan, Li Bing, Wu Xiuping. Application of carboxymethyl chitosan in tissue engineering of stomatology [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 447-452. |
| [8] | Zhong Wenjing, Zhou Zihan, Wang Haoyu, Li Shangyong, Xia Yujun. Preparation and in vitro evaluation of sustained-release nanoparticles loaded with sulfasalazine [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2461-2466. |
| [9] | Chen Di, Xue Yu, Tang Yu, Fei Xiaoming, Zhuang Qin, Zhou Wenwen, Lyu Demin, Shi Wentao, Zhang Zhijian, Zheng Wenjuan, Jiang Yu. Ecto-mesenchymal stem cell-conditioned medium lyophilized powder combined with fibrin glue to repair skin injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(15): 2350-2355. |
| [10] | Ma Yujiu, Zhang Xudong, Tan Jichun. Application status and prospect of menstrual blood-derived stem cells and their exosomes [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(15): 2427-2434. |
| [11] | Ma Hongfeng, Ren Qing, Cheng Wanmin, Wang Jiguo, Meng Qingyan. Effects of Sanhuang gel on wound healing, basic fibroblast growth factor and interleukin 17 levels of auricular skin defect in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1842-1847. |
| [12] | Ling Huajun, Cui Ruiwen, Wang Qiyou. 3D extracellular matrix hydrogel loaded with exosomes promotes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1900-1905. |
| [13] | Hu Jinlong, Quan Huahong, Wang Jingcheng, Zhang Pei, Zhang Jiale, Chen Pengtao, Liang Yuan. Effect of copper sulfide nanoparticles loaded thermosensitive hydrogel Pluronic F127 on infected wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1927-1931. |
| [14] | Ren Wenyan, Liu Xue, Wang Yiyu. Grophene-family nanomaterials in the treatment of periodontal disease: beneficial for osteogenic differentiation and reconstruction of periodontal support tissues [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1954-1960. |
| [15] | Zhang Yuqing, Wu Jun. Opportunities and challenges of COVID-19 therapy using mesenchymal stem cells and their exosomes [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1618-1625. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||