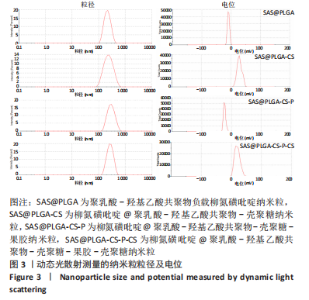
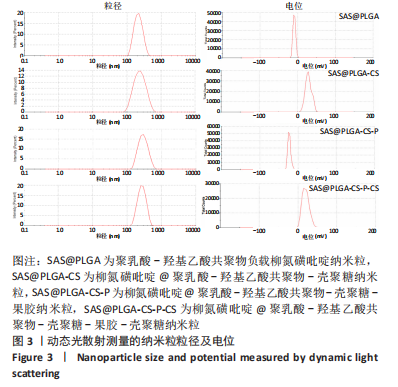
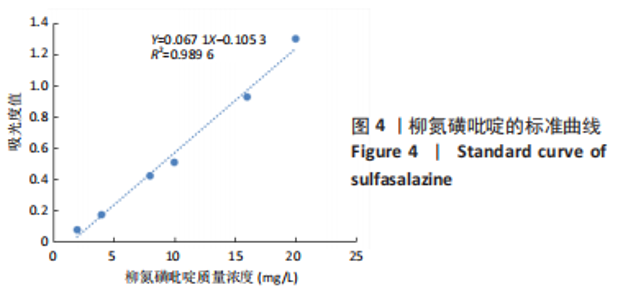
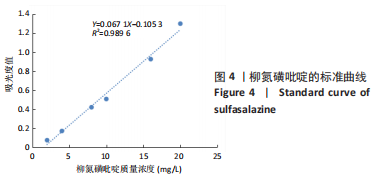
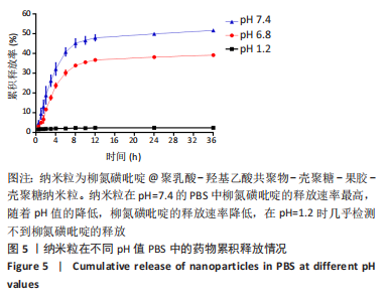
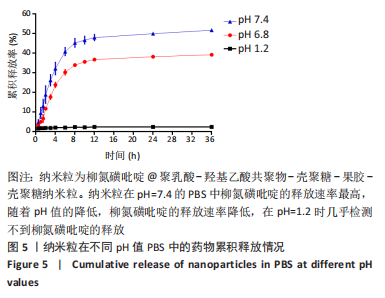
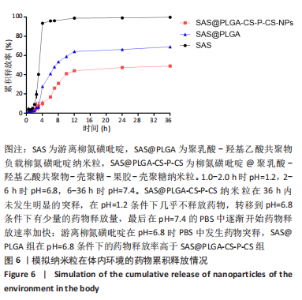
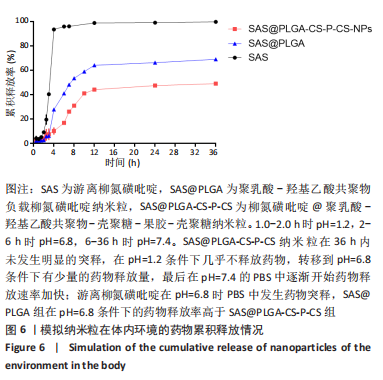
[1] UNGARO R, MEHANDRU S, ALLEN PB, et al.Ulcerative colitis. Lancet. 2017;389 (10080):1756-1770.
[2] DU L, HA C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49(4):643-654.
[3] KUCHARZIK T, KOLETZKO S, KANNENGIESSER K, et al. Ulcerative Colitis-Diagnostic and Therapeutic Algorithms. Dtsch Arztebl Int. 2020;117 (33-34):564-574.
[4] FEUERSTEIN JD, CHEIFETZ AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014;89(11):1553-1563.
[5] DOS SANTOS AM, CARVALHO SG, MENEGUIN AB, et al.Oral delivery of micro/nanoparticulate systems based on natural polysaccharides for intestinal diseases therapy: Challenges, advances and future perspectives. J Control Release. 2021;334:353-366.
[6] MITTAL R, PATEL AP, JHAVERI VM, et al. Recent advancements in nanoparticle based drug delivery for gastrointestinal disorders. Expert Opin Drug Deliv. 2018;15(3):301-318.
[7] TEONG B, LIN CY, CHANG SJ, et al. Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. J Mater Sci Mater Med. 2015;26(1):5357.
[8] BANSAL V, MALVIYA R, MALAVIYA T, et al.Novel prospective in colon specific drug delivery system. Polim Med. 2014;44(2):109-118.
[9] WANG QS, WANG GF, ZHOU J, et al.Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int J Pharm. 2016;515(1-2):176-185.
[10] RIVERA MC, PINHEIRO AC, BOURBON AI, et al.Hollow chitosan/alginate nanocapsules for bioactive compound delivery. Int J Biol Macromol. 2015;79:95-102.
[11] 范丽萍.壳寡糖/果胶静电自组装复合作用以及复合物负载BSA的研究[D].广州:华南理工大学,2019.
[12] WANG J, ZHANG C, GUO C, et al. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int J Mol Sci. 2019;20(22):5751.
[13] MARRAS-MARQUEZ T, PEÑA J, VEIGA-OCHOA MD. Robust and versatile pectin-based drug delivery systems. Int J Pharm. 2015;479(2):265-276.
[14] 徐仰仓,赵炳赫,衣丽霞,等.果胶的结构、提取及应用研究进展[J].福建技术师范学院学报,2021,39(5):437-442.
[15] DAS S. Pectin based multi-particulate carriers for colon-specific delivery of therapeutic agents. Int J Pharm. 2021;605:120814.
[16] BEUKEMA M, FAAS MM, DE VOS P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells. Exp Mol Med. 2020;52(9):1364-1376.
[17] 白春清,郑景霞,袁璐,等.果胶-壳聚糖多层修饰脂质体的制备及配方优化[J].食品工业科技,2016,37(21):49-53+59.
[18] LIU L, FISHMAN ML, KOST J, et al.Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials. 2003;24(19):3333-3343.
[19] 张文艳,李旷代,王强松,等.微/纳米口服结肠靶向给药系统在炎症性肠病治疗中的研究进展[J].天津中医药,2020,37(3):355-360.
[20] LI Y, ZHANG Y, ZHU CY. Pharmacokinetics and correlation between in vitro release and in vivo absorption of bio-adhesive pellets of panax notoginseng saponins. Chin J Nat Med. 2017;15(2):142-151.
[21] SOGIAS IA, WILLIAMS AC, KHUTORYANSKIY VV. Chitosan-based mucoadhesive tablets for oral delivery of ibuprofen. Int J Pharm. 2012; 436(1-2):602-610.
|