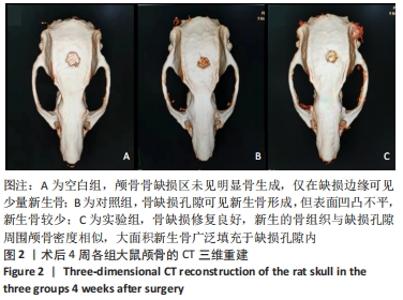
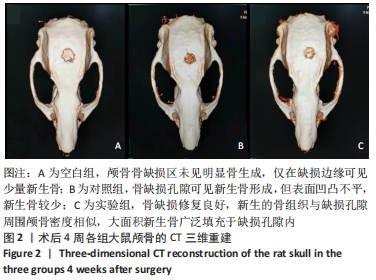
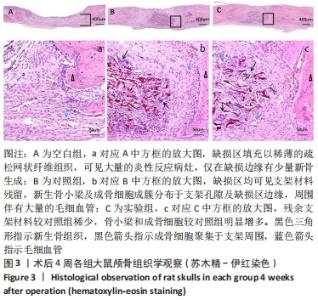
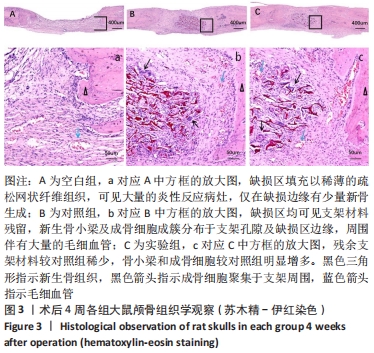
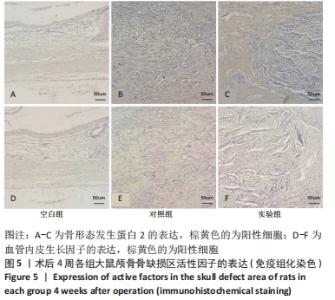
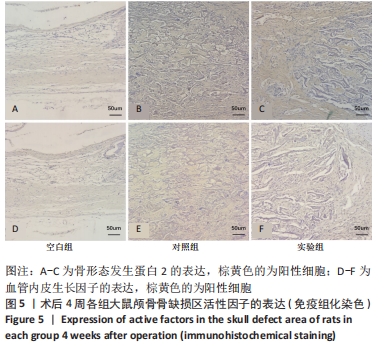
[1] 章晓云,陈跃平,宋世雷,等.丝素蛋白/壳聚糖复合支架有良好的细胞相容性与渗透性[J].中国组织工程研究,2020,24(16):2544-2550.
[2] 陈涛,付海洋,李岩,等.羟基磷灰石基复合骨修复材料研究进展[J].中国药事,2019,33(3):302-309.
[3] ZOU L, ZHANG Y, LIU X, et al. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf B Biointerfaces. 2019;178:222-229.
[4] 李军.骨碎补的研究概况[J].中药材,1999,22(5):263-266.
[5] 甘东浩,陈德强,冯蓬,等.基于网络药理学探讨骨碎补抗骨质疏松的物质基础及作用机制[J].中国实验方剂学杂志,2019,25(13): 186-191.
[6] 殷方明,肖涟波,张昀.骨碎补柚皮苷对炎症及骨作用的相关研究进展[J].中国骨伤,2015,28(2):182-186.
[7] ABOU FADEL R, SAMARANI R, CHAKAR C. Guided bone regeneration in calvarial critical size bony defect using a double-layer resorbable collagen membrane covering a xenograft: a histological and histomorphometric study in rats. Oral Maxillofac Surg. 2018;22(2):203-213.
[8] KRETLOW JD, MIKOS AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13(5):927-938.
[9] Kazemzadeh-Narbat M, Noordin S, Masri BA, et al. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J Biomed Mater Res B Appl Biomater. 2012;100(5):1344-1352.
[10] HONG MH, SON JS, KIM KM, et al. Drug-loaded porous spherical hydroxyapatite granules for bone regeneration. J Mater Sci Mater Med. 2011;22(2):349-355.
[11] 刘伟,赵劲民,苏伟,等.骨碎补总黄酮可促进兔骨髓间充质干细胞的增殖和分化[J].中国组织工程研究与临床康复,2011,15(32): 6021-6026.
[12] 李定,李悦,黄枫,等.骨碎补总黄酮在诱导膜技术中对骨缺损区域血管形成和成骨质量的影响[J].中华中医药杂志,2019,34(11): 5086-5089.
[13] 李晋玉,俞兴,姜俊杰,等.骨碎补总黄酮联合纳米骨材料促进MC3T3-E1细胞的增殖分化[J].中国组织工程研究,2020,24(7): 1030-1036.
[14] 刘小坡,冯云波,曹国龙,等.柚皮苷对老年大鼠骨质疏松性骨折愈合的影响[J]. 中国临床药理学杂志,2020,36(9):1117-1120.
[15] 刘冲,曹慧,杨彩彩,等.柚皮苷调控miR-199a-5p/ECE1分子轴促进骨损伤修复[J].昆明医科大学学报,2020,41(6):32-38.
[16] 赵志虎,孟新民,孙晓雷,等.柚皮苷对去势大鼠骨折骨痂血管发生的影响及其机制[J].中华骨科杂志,2016,36(3):177-183.
[17] HE X, LIU Y, YUAN X, et al. Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/hydroxyapatite scaffolds. PLoS One. 2014;9(8):e104061.
[18] CAO Z, JIANG D, YAN L, et al. In vitro and in vivo drug release and antibacterial properties of the novel vancomycin-loaded bone-like hydroxyapatite/poly amino acid scaffold. Int J Nanomedicine. 2017;12: 1841-1851.
[19] JI Y, WANG L, WATTS DC, et al. Controlled-release naringin nanoscaffold for osteoporotic bone healing. Dent Mater. 2014;30(11):1263-1273.
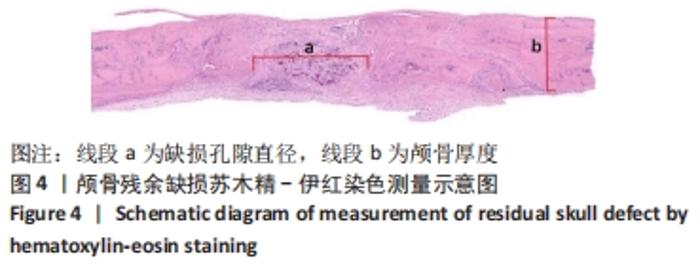
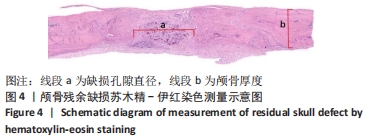
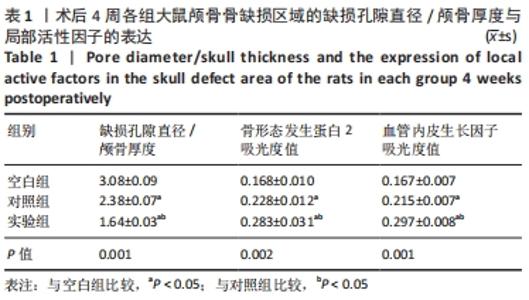
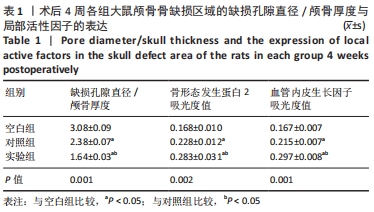
|