Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (4): 521-527.doi: 10.12307/2022.086
Previous Articles Next Articles
Biosafety evaluation of three-dimensional printed gelatin/sodium alginate/58S bioactive glass scaffolds for bone defect repair
Tan Guozhong1, Tu Xinran1, Guo Liyang1, Zhong Jialin1, Zhang Yang2, Jiang Qianzhou1
- 1Department of Endodontics, Affiliated Stomatology Hospital of Guangzhou Medical University, Guangzhou Key Laboratory of Basic and Applied Research of Oral Regenerative Medicine, Guangzhou 510182, Guangdong Province, China; 2Guangzhou ZhongDa Medical Equipment Company Limited, Guangzhou 510300, Guangdong Province, China
-
Received:2020-10-20Revised:2020-10-22Accepted:2021-01-07Online:2022-02-08Published:2021-11-03 -
Contact:Jiang Qianzhou, Chief physician, Department of Endodontics, Affiliated Stomatology Hospital of Guangzhou Medical University, Guangzhou Key Laboratory of Basic and Applied Research of Oral Regenerative Medicine, Guangzhou 510182, Guangdong Province, China -
About author:Tan Guozhong, Master candidate, Physician, Department of Endodontics, Affiliated Stomatology Hospital of Guangzhou Medical University, Guangzhou Key Laboratory of Basic and Applied Research of Oral Regenerative Medicine, Guangzhou 510182, Guangdong Province, China -
Supported by:Science and Technology Planning Project of Guangdong Province, No. 2018B050502012 (to JQZ)
CLC Number:
Cite this article
Tan Guozhong, Tu Xinran, Guo Liyang, Zhong Jialin, Zhang Yang, Jiang Qianzhou. Biosafety evaluation of three-dimensional printed gelatin/sodium alginate/58S bioactive glass scaffolds for bone defect repair[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 521-527.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
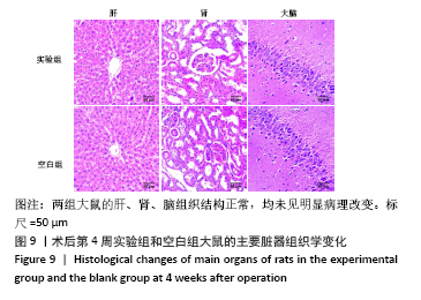
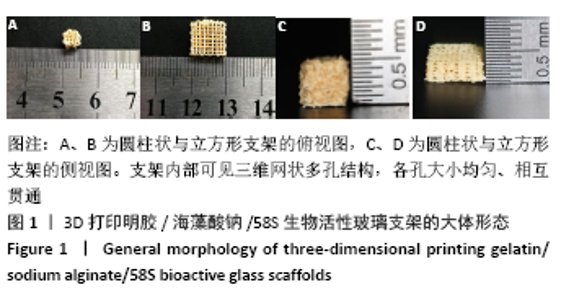
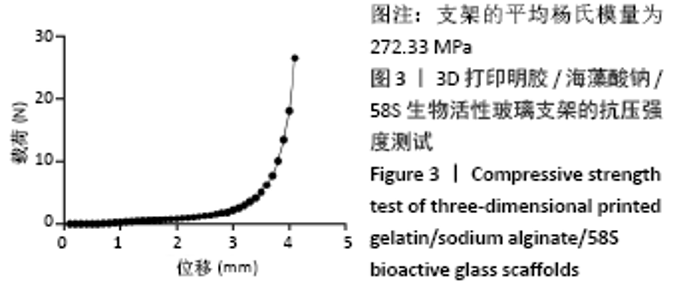
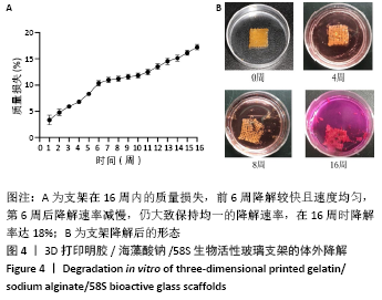
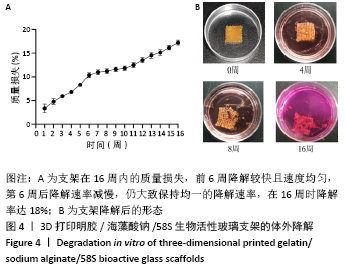
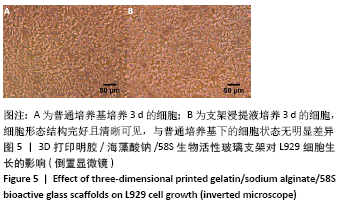
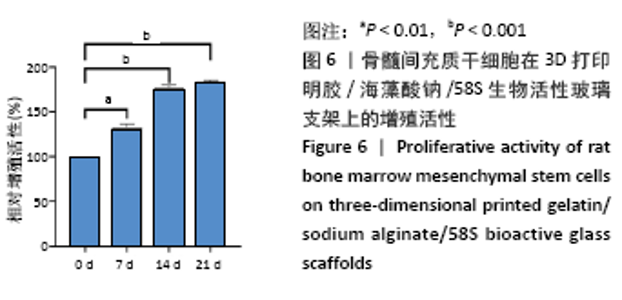
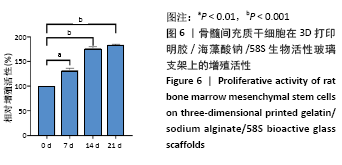
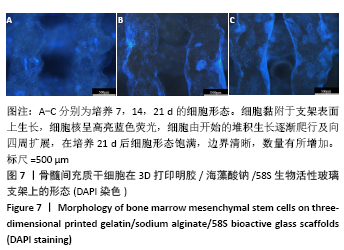
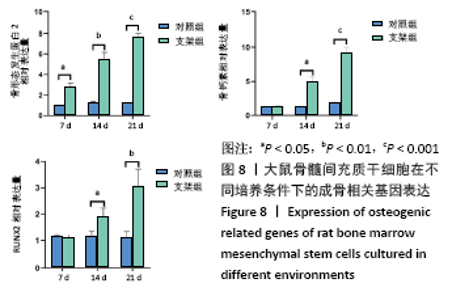
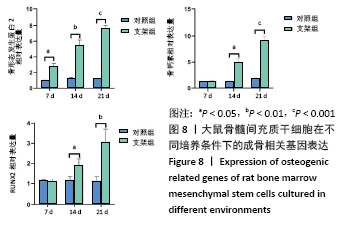
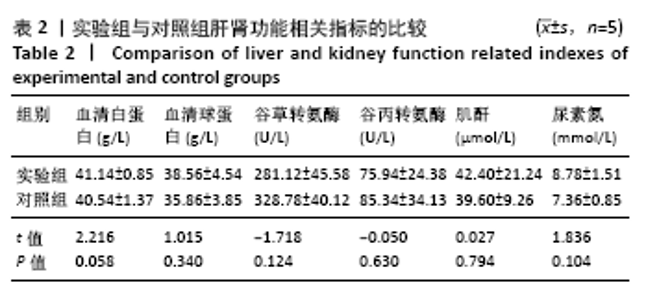
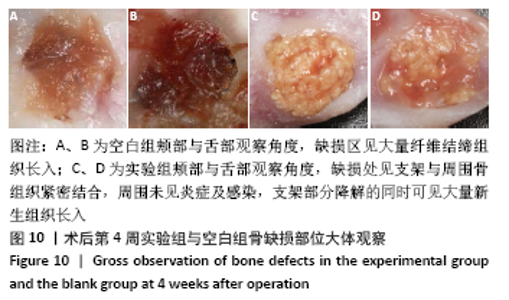
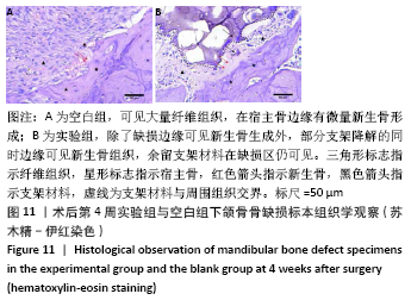
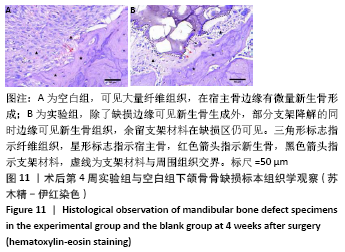
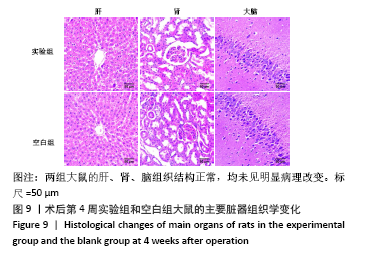
大鼠肝、肾和脑组织的苏木精-伊红染色观察,见图9,正常结构单位清晰可见,细胞形态正常,均未见病理性改变。 术后4周,两组骨缺损区颊舌侧愈合良好,无红肿、感染和坏死情况发生,空白组缺损区见纤维结缔组织长入填充,实验组部分材料发生降解,余留材料与周围组织有机结合,表面见大量纤维组织包绕,见图10,表明支架材料具有良好的组织相容性。大鼠下颌骨骨缺损标本苏木精-伊红染色显示,实验组支架材料未完全降解,可见内部孔隙轮廓,新生骨连接宿主骨与余留支架材料,新生骨组织周围可见少量成骨细胞浸润及炎性细胞浸润;空白组宿主骨边缘见少量新生骨及大量纤维组织,见图11。 2.4 支架材料的生物相容性 体外细胞毒性实验、增殖实验与体内骨缺损修复实验显示,3D打印明胶/海藻酸钠/58S生物活性玻璃支架具有良好的细胞相容性与组织相容性。 "
| [1] HWANG KS, CHOI JW, KIM JH, et al. Comparative Efficacies of Collagen-Based 3D Printed PCL/ PLGA/β-TCP Composite Block Bone Grafts and Biphasic Calcium Phosphate Bone Substitute for Bone Regeneration. Materials (Basel). 2017;10(4):421. [2] MANO T, AKITA K, FUKUDA N, et al. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J Biomed Mater Res B Appl Biomater. 2020;108(4):1450-1459. [3] LEE Y, CHAN Y, HSIEH S, et al. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int J Mol Sci. 2019; 20(20):5015. [4] ZHU T, CUI Y, ZHANG M, et al. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact Mater. 2020;5:584-601. [5] WANG C, HUANG W, ZHOU Y, et al. 3D printing of bone tissue engineering scaffolds.Bioact Mater. 2020;5: 82-91. [6] 刘洋,王欢,朱晔,等.骨组织工程支架材料研究进展[J].临床口腔医学杂志,2019,35(10):637-639. [7] 范海霞,王宏,程焕芝,等.3D打印HAP-GEL支架复合BMSCs和HUVECs细胞修复兔颅骨缺损[J].临床口腔医学杂志,2020,36(4):199-202. [8] ASHAMMAKHI N, HASAN A, KAARELA O, et al. Advancing Frontiers in Bone Bioprinting. Adv Healthc Mater. 2019;8:e1801048. [9] ZHANG L, YANG G, JOHNSON B, et al. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16-33. [10] SOORIYAARACHCHI D, MINIÈRE H, MAHARUBIN S, et al. Hybrid Additive Microfabrication Scaffold Incorporated with Highly Aligned Nanofibers for Musculoskeletal Tissues. Tissue Eng Regen Med. 2019;16:29-38. [11] GU Q, HAO J, LU Y, et al. Three-dimensional bio-printing. Sci China Life Sci. 2015;58:411-419. [12] PUROHIT S, SINGH H, BHASKAR R, et al. Gelatin-alginate-cerium oxide nanocomposite scaffold for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;116:111111 [13] ZHENG A, CAO L, LIU Y, et al. Biocompatible silk/calcium silicate/sodium alginate composite scaffolds for bone tissue engineering. Carbohydr Polym. 2018;199:244-255. [14] YU H, ZHANG X, SONG W, et al. Effects of 3-dimensional Bioprinting Alginate/ Gelatin Hydrogel Scaffold Extract on Proliferation and Differentiation of Human Dental Pulp Stem Cells. J Endod. 2019;45:706-715. [15] YE Q, ZHANG Y, DAI K, et al. Three dimensional printed bioglass/gelatin/alginate composite scaffolds with promoted mechanical strength, biomineralization, cell responses and osteogenesis. J Mater Sci Mater Med. 2020;31(9):77. [16] 吕孝帅,李正茂,杨雪超.生物活性玻璃58 S/丝素蛋白膜促进人牙髓干细胞增殖与分化[J].牙体牙髓牙周病学杂志,2015(10):577-583. [17] YAO Q, LIU H, LIN X, et al. 3D Interpenetrated Graphene Foam/58S Bioactive Glass Scaffolds for Electrical-Stimulation-Assisted Differentiation of Rabbit Mesenchymal Stem Cells to Enhance Bone Regeneration. J Biomed Nanotechnol. 2019;15:602-611. [18] DING Y, LI W, MÜLLER T, et al. Electrospun Polyhydroxybutyrate/Poly(ε-caprolactone)/58S Sol-Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2016;8:17098-17108. [19] WU J, MIAO G, ZHENG Z, et al. 3D printing mesoporous bioactive glass/sodium alginate/gelatin sustained release scaffolds for bone repair. J Biomater Appl. 2019;33:755-765. [20] YANG S, XU S, ZHOU P, et al. Siliceous mesostructured cellular foams/poly (3-hydroxybutyrate–co-3 -hydroxyhexanoate) composite biomaterials for bone regeneration. Int J Nanomedicine. 2014;9:4795-4807. [21] KIM J, PARK J, MUTHUKUMAR T, et al. Accelerating bone defects healing in calvarial defect model using 3D cultured bone marrow-derived mesenchymal stem cells on demineralized bone particle scaffold. J Tissue Eng Regen Med. 2020;14:563-574. [22] 潘朝晖,栾兆新,高朋.两种交联剂对β-磷酸三钙明胶复合骨支架理化及生物学性能影响的比较[J].中国组织工程研究,2018,22(6):833-839. [23] KIKUCHI M, MATSUMOTO H, YAMADA T, et al. Glutaraldehyde cross-linked hydroxyapatite/collagen self-organized nanocomposites. Biomaterials. 2004;25:63-69. [24] PENG Y, GLATTAUER V, RAMSHAW J. Stabilisation of Collagen Sponges by Glutaraldehyde Vapour Crosslinking.Int J Biomater. 2017;2017:8947823. [25] SUESCA E, DIAS A, BRAGA M, et al. Multifactor analysis on the effect of collagen concentration, cross-linking and fiber/pore orientation on chemical, microstructural, mechanical and biological properties of collagen type I scaffolds. Mater Sci Eng C Mater Biol Appl. 2017;77:333-341. [26] SUN X, MA C, GONG W, et al. Biological properties of sulfanilamide-loaded alginate hydrogel fibers based on ionic and chemical crosslinking for wound dressings.Int J Biol Macromol. 2020;157:522-529. [27] GHADERI G, SAHEBGHADAM L, KORDI T, et al. The enhancement of differentiating adipose derived mesenchymal stem cells toward hepatocyte like cells using gelatin cryogel scaffold. Biochem Biophys Res Commun. 2017;491:1000-1006. [28] SIVASHANKARI P, MOORTHI A, ABUDHAHIR K, et al. Preparation and characterization of three-dimensional scaffolds based on hydroxypropyl chitosan-graft-graphene oxide. Int J Biol Macromol. 2018;110:522-530. [29] 王闯建,刘宏建,张春霖,等.喷墨全包芯骨支架材料的制备及生物安全性研究[J].中华实验外科杂志,2019,36(5):915-918 [30] CHEN Y, KAWAZOE N, CHEN G. Preparation of dexamethasone-loaded biphasic calcium phosphate nanoparticles/collagen porous composite scaffolds for bone tissue engineering. Acta Biomater. 2018;67:341-353. [31] HOPPE A, GÜLDAL N, BOCCACCINI A. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757-2774. [32] GERHARDT L, BOCCACCINI A. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials (Basel). 2010;3(7):3867-3910. |
| [1] | Xue Yadong, Zhou Xinshe, Pei Lijia, Meng Fanyu, Li Jian, Wang Jinzi . Reconstruction of Paprosky III type acetabular defect by autogenous iliac bone block combined with titanium plate: providing a strong initial fixation for the prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1424-1428. |
| [2] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [3] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [4] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [5] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [6] | Liu Yingsong, Guo Xiaopeng, Wei Mingzhu. Transforming growth factor beta 3 and alginate hydrogel complex on the repair of articular cartilage defects of the knee [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2504-2509. |
| [7] | Yuan Yihang, Xu Menghan, Niu Xufeng. Gelatin collagen composite hydrogel and inducible factor regulate differentiation of rat bone marrow mesenchymal stem cells into hepatocyte-like cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2510-2515. |
| [8] | Li Mingxin, Li Jun, Wang Wenchao, Song Ping, Lei Haoyuan, Gui Xingyu, Zhang Chengyun, Zhou Changchun, Liu Lei. Cell-carrying porous methacrylate anhydride gelatin three-dimensional scaffolds and their effects on cell behavior [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2532-2539. |
| [9] | Wei Zhangao, Xu Linghan, Wu Zichen, Tang Hao, Chen Jialong. Application of inorganic nonmetallic artificial bone materials in vivo [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2584-2589. |
| [10] | Liu Xiaolin, Liu Shutai, Han Xiaoqian, Mu Xinyue. Topical simvastatin administration in the treatment of periodontitis: effect of loading on sustained drug release system or biomaterial scaffold system [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2596-2601. |
| [11] | Yang Xue, Wang Baoqun, Jiang Xiaowen, Zou Shengcan, Ming Jinfa, Lin Shasha. Preparation and properties of biodegradable plant polysaccharide hemostatic microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2487-2491. |
| [12] | Gao Xixin, Wang Xi, Fan Xuhui, Cui Yi, Yang Wei, Zhao Yunzhuan. Platelet-rich fibrin combined with induced bone matrix repairs bone defects around oral implants in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(14): 2207-2213. |
| [13] | Li Shijie, Ma Liqiong, Xiong Xianmei, Zhang Yan, Chen Zijie, Feng Junming, Gao Yijia, Zeng Zhanpeng. Effect of panax notoginseng saponins on platelet-rich plasma promoting bone defect healing in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(14): 2155-2160. |
| [14] | Liao Xinyu, Wang Fuke, Li Yanlin, Wang Guoliang, Yang Guiran, Hou Jianfei, Yang Tengyun, Zhong Ruiying. Tissue-engineered bone constructed by co-culture of vascular endothelial cells, adipose derived stem cells, and partially deproteinized biological bone to repair jaw defects [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(13): 2027-2033. |
| [15] | Zhao Yuexin, Chen Bin. Progress of macrophage polarization in immunology of bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(13): 2120-2126. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||