Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (13): 2027-2033.doi: 10.12307/2022.328
Previous Articles Next Articles
Tissue-engineered bone constructed by co-culture of vascular endothelial cells, adipose derived stem cells, and partially deproteinized biological bone to repair jaw defects
Liao Xinyu, Wang Fuke, Li Yanlin, Wang Guoliang, Yang Guiran, Hou Jianfei, Yang Tengyun, Zhong Ruiying
- Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China
-
Received:2021-03-10Revised:2021-03-15Accepted:2021-04-23Online:2022-05-08Published:2021-12-20 -
Contact:Wang Fuke, MD, Professor, Chief physician, Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China -
About author:Liao Xinyu, Master candidate, Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China -
Supported by:General Project of Applied Basic Research Joint Special Fund of Yunnan Provincial Department of Science and Technology-Kunming Medical University, No. 201701UH00095 (to WFK)
CLC Number:
Cite this article
Liao Xinyu, Wang Fuke, Li Yanlin, Wang Guoliang, Yang Guiran, Hou Jianfei, Yang Tengyun, Zhong Ruiying. Tissue-engineered bone constructed by co-culture of vascular endothelial cells, adipose derived stem cells, and partially deproteinized biological bone to repair jaw defects[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(13): 2027-2033.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
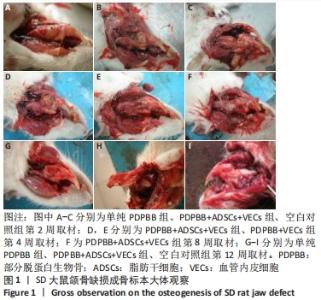
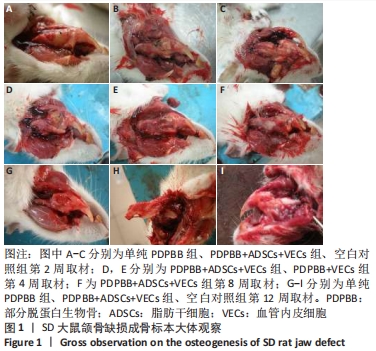
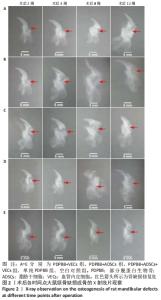
2.1 一般情况观察 2只SD大鼠术后死亡;3只出现骨缺损处感染,移植物排出,予以补做实验;其余动物第1天开始进食,饮食、活动正常,术后第1周切口肿胀,2周后完全愈合。 2.2 大体观察结果 第2周取材,单纯PDPBB组可见大量纤维组织包绕植入的组织工程骨,连接紧密,组织工程骨还有一定的活动度,见图1A;PDPBB+ADSCs+VECs组可见纤维组织长入骨支架的孔隙,孔隙减小,见图1B;空白对照组可见纤维组织已经封闭骨缺损,去除软组织后骨缺损依然存在,见图1C。第4周取材,PDPBB+ADSCs+VECs组可见纤维组织包绕连接植入的组织工程骨,植入材料不能移动,见图1D;PDPBB+VECs组可见周围肌肉及纤维组织明显增厚,把组织工程骨和下颌骨连接成一体,组织工程骨血供较其他组丰富,植入材料不能移动,见图1E。第8周取材,可见多数组织仍以纤维连接为主,PDPBB+ADSCs+VECs组去除周围组织,可见组织工程骨空隙已经消失,与下颌骨骨性连接,表面部分材料已皮质化,见图1F。第12周取材,各组均已和下颌骨骨性连接,单纯PDPBB组可见材料部分吸收,材料大体还保留有原来形态,见图1G;PDPBB+ADSCs+VECs组材料形态和正常下颌骨相似,表面已皮质化,材料已经大部分吸收,仅有少量未被取代,见图1H;空白对照组骨缺损未被修复,骨断裂部分已经被骨皮质封闭,形成半圆形骨缺损区,见图1I。"
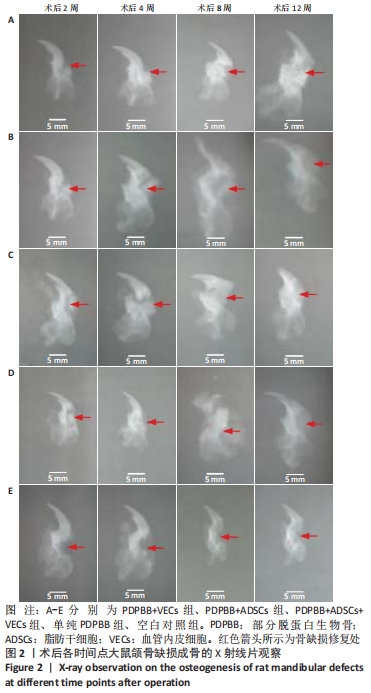
2.3 颌骨缺损成骨的X射线片检查结果 ①PDPBB+VECs组:第2周支架材料与下颌骨缺损之间的缝隙较宽,周围有新生骨痂生成;第4-8周骨痂逐渐增多,第12周较前相比组织工程骨的骨密度明显增加,与正常骨质相比已基本相同,见图2A。②PDPBB+ADSCs组:第2周骨缺损周围骨痂开始生成,随时间延长逐渐增加,植入生物骨和缺损之间缝隙减小,第8周和第12周缝隙之间消失,第12周时支架材料与下颌骨缺损之间缝隙明显减淡,但支架材料大体形态依然存在,见图2B。③PDPBB+ADSCs+VECs组:第4-12周骨缺损周围骨痂生成随时间延长逐渐增加,植入生物骨和缺损之间缝隙减小,第8周已经骨性联合,骨缝已经消失;第12周骨密度明显增加,骨形态已经基本恢复正常,见图2C。④单纯PDPBB组:第2周可见支架材料与下颌骨缺损之间的缝隙较宽,周围有新生骨痂生成;第4-12周骨痂逐渐增多,第12周时支架材料骨密度较前减轻,与正常骨质对比明显,见图2D。⑤空白对照组:第2周颌骨体部有长方形骨缺损,未见明显骨痂;第4周在骨缺损周边少许骨痂出现;第8周和第12周骨缺损面积减小,边缘有骨痂修复,骨皮质已经封闭缺损端,仅剩下半圆或三角形缺损,见图2E。"
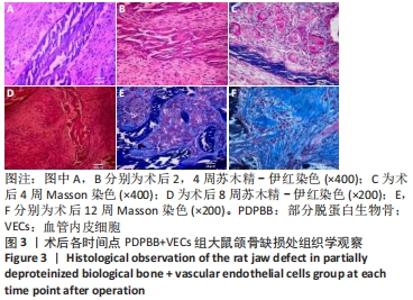
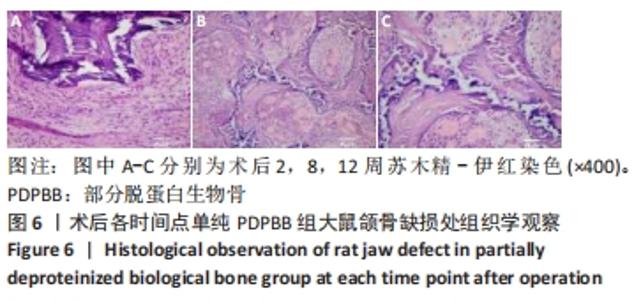
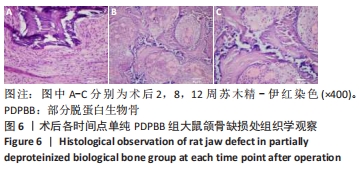
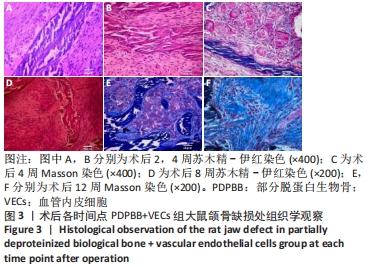
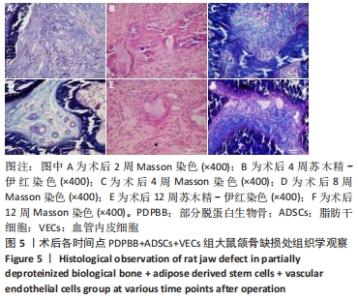
2.4 颌骨缺损成骨的组织学观察结果 PDPBB+VECs组:第2周支架材料周围出现大量多形性细胞,核大,排列不规则,部分细胞为多核巨细胞,侵蚀入支架材料内部,外层为层状排列的梭形成纤维细胞,见图3A;第4周支架材料周围多核破骨细胞和巨噬细胞增多,大量细胞侵入支架材料内部,骨支架内部出现大量细胞结构,见图3B,支架材料被周围多核破骨细胞和巨噬细胞侵蚀,可见多个月牙形改变,周围大量新生血管形成,见图3C;第8周支架材料周围改变外,还可见大量的小血管形成,见图3D;第12周支架材料内部部分骨支架吸收,梭形成纤维细胞消失,出现大量多形性髓腔细胞成分,见图3E,支架材料部分吸收,周围梭形成纤维样细胞分泌大量胶原,平行或螺旋形排列,编织成网状,见图3F。"
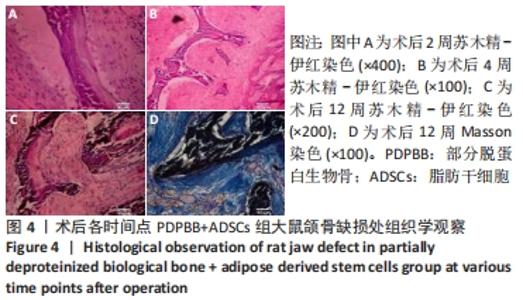
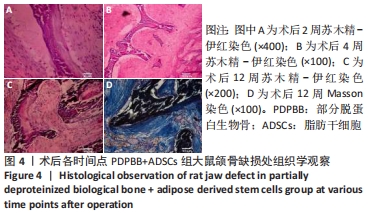
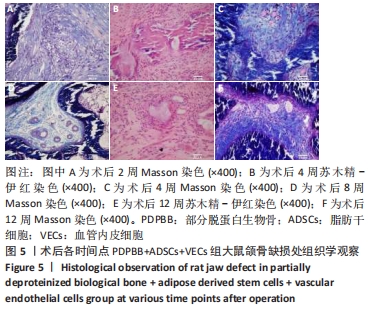
PDPBB+ADSCs+VECs组:第2周有大量软组织长入组织工程骨孔隙,大部分为梭形成纤维细胞,胶原分泌少,少量淋巴细胞浸润,见图5A;第4周支架材料周围存在大量的破骨细胞,骨支架材料被分割降解,骨支架内部出现细胞结构,周围成纤维细胞排列紊乱,骨支架周围形成大量的骨样物质,见图5B;支架材料降解、碎裂,成纤维样细胞长入加快支架材料降解;成纤维细胞胶原分泌量增多,排列紊乱;大量新生小血管形成,见图5C;第8周纤维组织纤维包裹支架材料,材料降解形成多个月牙形凹痕,软组织中出现多个新生小血管,局部出现髓腔化改变,见图5D;第12周支架材料部分降解完毕,取代为新的成骨中心,周围存在大量的巨噬细胞和新生血管网,见图5E;支架材料周围胶原组织堆积形成新的骨小梁结构,骨细胞包埋进胶原内部,大量骨支架被降解取代,见图5F。"
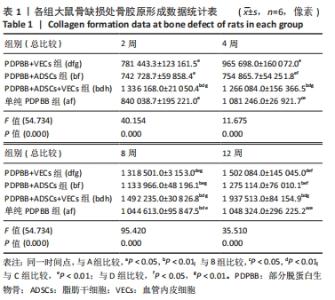
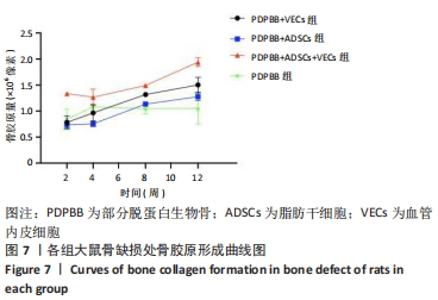
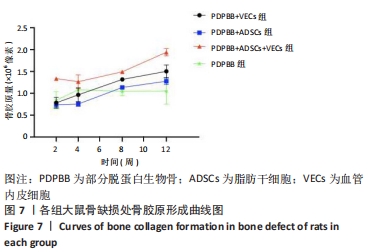
统计学分析各分组差异有非常显著性意义(F=54.734,P < 0.01);PDPBB+ADSCs组与单纯PDPBB组比较差异无显著性意义(P=0.607 > 0.05),PDPBB+VECs组与PDPBB+ADSCs组比较差异有显著性意义(P=0.011 < 0.05);其余各组两两比较差异均有非常显著性意义(P < 0.01)。第2周数据分析各组差异有非常显著性意义(F=40.154,P < 0.01);PDPBB+ADSCs+VECs组与其他组两两比较差异有非常显著性意义(P < 0.01),其余各组两两比较差异无显著性意义(P > 0.05)。第4周数据分析各组差异有非常显著性意义(F=11.675,P < 0.01),PDPBB+VECs组与单纯PDPBB组和PDPBB+ADSCs组比较差异无显著性意义 (P > 0.05),单纯PDPBB组与PDPBB+ADSCs组两两比较差异有显著性意义(P=0.047 < 0.05);其余各组两两比较差异均有非常显著性意义(P < 0.01)。第8周数据分析各组差异有显著性意义(F=95.420,P < 0.01),各组两两比较差异均有非常显著性意义(P < 0.01)。第12周数据分析各组差异有非常显著性意义(F=35.510,P < 0.01);PDPBB+ADSCs组与PDPBB+VECs组和单纯PDPBB组两两比较差异有显著性意义(P < 0.05),其余各组两两比较差异均有非常显著性意义(P < 0.01),见表1。"

| [1] SAADEH PB, MEHRARA BJ, STEINBRECH DS, et al. Mechanisms of fibroblast growth factor-2 modulation of vascular endothelial growth factor expression by osteoblastic cells. Endocrinology. 2000;141(6):2075-2083. [2] GRIFFITH LG, NAUGHTON G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295(5557):1009-1014. [3] 中华人民共和国科学技术部. 关于善待实验动物的指导性意见[OL]. 2006-09-30.[2008-08-15].http://www.most.gov.cn/zfwj/zfwj2006/200512/t20051214_54389.htm [4] 王福科,刘流,李彦林,等.骨髓基质干细胞与PDPB体外构建组织工程骨的适宜条件[J].中国组织工程研究与临床康复,2008,12(33):6401-6405. [5] 王福科,张红,李彦林,等.联合培养血管内皮细胞与脂肪干细胞构建组织工程骨异位成骨[J].中国修复重建外科杂志,2019,33(10):1310-1319. [6] JORGE RS, JORGE J JR, LUZ JG. Reconstruction of a mandibular critical-sized defect using iliac graft in rats. Implant Dent. 2006;15(3):282-289. [7] KOSTOPOULOS L, KARRING T. Guided bone regeneration in mandibular defects in rats using a bioresorbable polymer. Clin Oral Implants Res. 1994; 5(2):66-74. [8] SWANSON WB, OMI M, ZHANG Z, et al. Macropore design of tissue engineering scaffolds regulates mesenchymal stem cell differentiation fate. Biomaterials. 2021;272:120769. [9] LAMMERT E, CLEAVER O, MELTON D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294(5542):564-567. [10] GUERRA A, BELINHA J, NATAL JORGE R. A preliminary study of endothelial cell migration during angiogenesis using a meshless method approach. Int J Numer Method Biomed Eng. 2020;36(11):e3393. [11] RONG Q, LI S, ZHOU Y, et al. A novel method to improve the osteogenesis capacity of hUCMSCs with dual-directional pre-induction under screened co-culture conditions. Cell Prolif. 2020;53(2):e12740. [12] DECKERS MM, VAN BEZOOIJEN RL, VAN DER HORST G, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143(4):1545-1553. [13] KIM SD, YI JM, PARK MT. Irradiated endothelial cells modulate the malignancy of liver cancer cells. Oncol Lett. 2019;17(2):2187-2196. [14] WEIJTS B, SHAKED I, GINSBERG M, et al. Endothelial struts enable the generation of large lumenized blood vessels de novo. Nat Cell Biol. 2021; 23(4):322-329. [15] LANGE L, MORGAN M, SCHAMBACH A. The hemogenic endothelium: a critical source for the generation of PSC-derived hematopoietic stem and progenitor cells. Cell Mol Life Sci. 2021 Feb 9. doi: 10.1007/s00018-021-03777-y. [16] PEICHEV M, NAIYER AJ, PEREIRA D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952-958. [17] PESCE M, ORLANDI A, IACHININOTO MG, et al. Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissues. Circ Res. 2003;93(5):e51-62. [18] BAIER J, GWELLEM AC, HAASE R, et al. Co-Culture of Peripheral Blood Mononuclear Cells and Endothelial Colony Forming Cells from Cord Blood of Preterm Born Babies. Methods Mol Biol. 2021;2269:107-124. [19] RÜGER BM, BUCHACHER T, DAUBER EM, et al. De novo Vessel Formation Through Cross-Talk of Blood-Derived Cells and Mesenchymal Stromal Cells in the Absence of Pre-existing Vascular Structures. Front Bioeng Biotechnol. 2020;8:602210. [20] ZHAO X, HAN XS, ZHOU QZ, et al. Repair of Bone Defects With Endothelial Progenitor Cells and Bone Marrow-Derived Mesenchymal Stem Cells With Tissue-Engineered Bone in Rabbits. Ann Plast Surg. 2020;85(4):430-436. [21] MUSCHLER GF, NITTO H, BOEHM CA, et al. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19(1):117-125. [22] 谭洪波,杨柳,段小军,等.骨组织工程支架体外血管化初步研究[J].重庆医学,2007,36(6):499-501. [23] LIU Y, DULCHAVSKY DS, GAO X, et al. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res. 2006;136(2):336-341. [24] 黄艳欣,杜雅菊,李宝杰.肝细胞与骨髓间充质干细胞共同培养时的肝细胞功能[J].世界华人消化杂志,2004,12(5):1129-1131. [25] MURAKAMI S, IJIMA H, ONO T, et al. Development of co-culture system of hepatocytes with bone marrow cells for expression and maintenance of hepatic functions. Int J Artif Organs. 2004;27(2):118-126. [26] QIHAO Z, XIGU C, GUANGHUI C, et al. Spheroid formation and differentiation into hepatocyte-like cells of rat mesenchymal stem cell induced by co-culture with liver cells. DNA Cell Biol. 2007;26(7):497-503. [27] KIM SJ, KIM BS, RYU SW, et al. Hematopoietic differentiation of embryoid bodies derived from the human embryonic stem cell line SNUhES3 in co-culture with human bone marrow stromal cells. Yonsei Med J. 2005; 46(5):693-639. [28] BOROUJENI MB, SALEHNIA M, VALOJERDI MR, et al. Comparison of gene expression profiles in erythroid-like cells derived from mouse embryonic stem cells differentiated in simple and co-culture systems. Am J Hematol. 2008;83(2):109-115. |
| [1] | Zhang Yujie, Yang Jiandong, Cai Jun, Zhu Shoulei, Tian Yuan. Mechanism by which allicin inhibits proliferation and promotes apoptosis of rat vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1080-1084. |
| [2] | Li Shijie, Ma Liqiong, Xiong Xianmei, Zhang Yan, Chen Zijie, Feng Junming, Gao Yijia, Zeng Zhanpeng. Effect of panax notoginseng saponins on platelet-rich plasma promoting bone defect healing in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(14): 2155-2160. |
| [3] | Liu Liu, Zhou Qingzhu, Gong Zhuo, Liu Boyan, Yang Bin, Zhao Xian. Characteristics and manufacturing techniques of collagen/inorganic materials for constructing tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 607-613. |
| [4] | Jiang Tao, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Ma Chuang, Wei Qin. Platelet-derived growth factor BB induces bone marrow mesenchymal stem cells to differentiate into vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3937-3942. |
| [5] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [6] | Zhang Shengmin, Cao Changhong, Liu Chao. Adipose-derived stem cells integrated with concentrated growth factors prevent bisphosphonate-related osteonecrosis of the jaws in SD rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2982-2987. |
| [7] | Liu Tao, Zhang Nini, Huang Guilin . Relationship between extracellular vesicles and radiation-induced tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2121-2126. |
| [8] | Zhang Jian, Chen Miao, Li Weixin, Ye Yichao, Xu Huiyou, Ma Ke, Chen Xuyi, Sun Hongtao, Zhang Sai. Collagen/heparin sulfate scaffolds loaded with brain-derived neurotrophic factor promote neurological and locomotor function recovery in rats after traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5538-5544. |
| [9] | Chen Jia, Yang Yiqiang, Hu Chen, Chen Qi, Zhao Tian, Yong Min, Ma Dongyang, Ren Liling. Fabrication of prevascularized osteogenic differentiated cell sheet based on human bone marrow mesenchymal stem cells and human umbilical vein endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 4934-4940. |
| [10] | Sun Xirao, Wang Chengyue, Zhao Yuan, Zhang Zhenbao. In vitro corrosion and in vivo biosafety of pure magnesium film [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(16): 2578-2584. |
| [11] | Lin Liulan, Zhou Jianyong. Application status of 3D printed polyetheretherketone and its composite in bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(10): 1622-1628. |
| [12] | Sun An, Bi Xiangyu, Han Xiangzhen, Zhou Qiqi, He Huiyu. Effects of vascular endothelial growth factor combined with platelet-derived growth factor-BB on the angiogenic and proliferative abilities of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(1): 1-6. |
| [13] | Zhan Xiaoshu, Luo Huina, Luo Dongzhang, Chen Shengfeng, Wang Bingyun, Bai Yinshan, Chen Zhisheng, Liu Canying, Ji Huiqin. Effects of exosomes derived from canine umbilical cord mesenchymal stem cells on proliferation, migration and apoptosis of vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(29): 4637-4643. |
| [14] | Ding Yan, Meng Biying, Xiang Guangda. Establishment and identification of a mouse model of vascular endothelial cell knockout DEPTOR gene [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(23): 3698-3704. |
| [15] | Yan Jun-ling, Di Guo-hu, Ding Hong, Zhang Wei, Liu Jia-qi, Tang Su-yang. Auto-adipose stem cells facilitate autologous fat transfer for breast augmentation [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(5): 878-885. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||