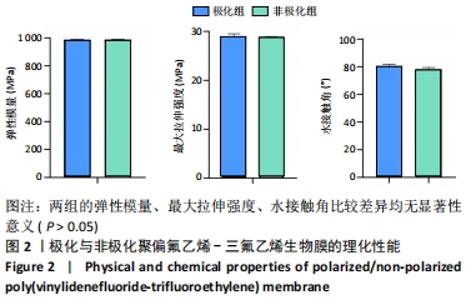
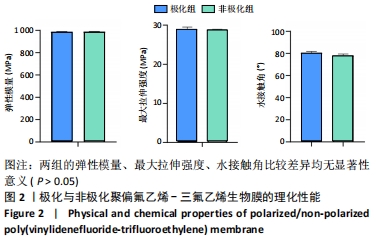
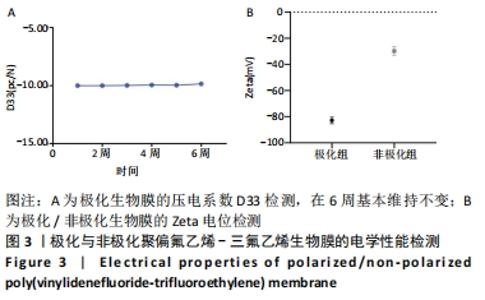
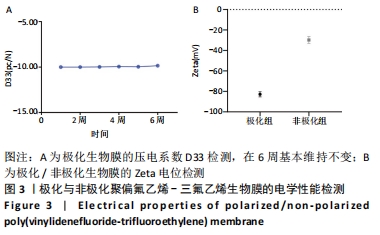
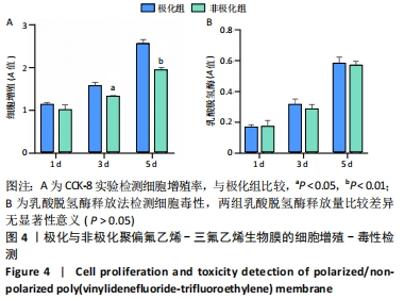
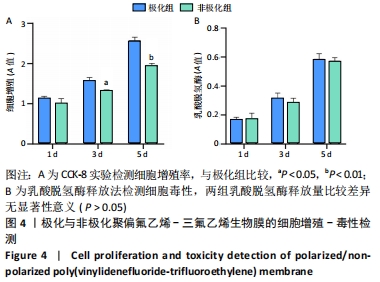
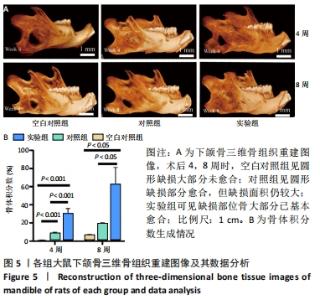
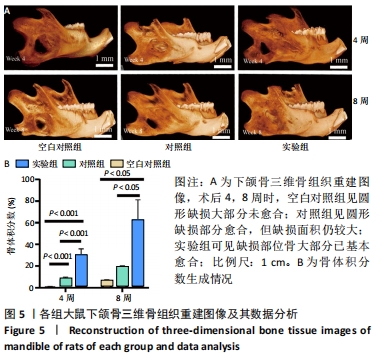
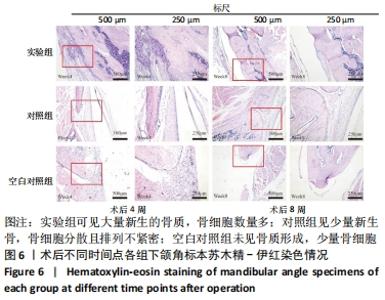
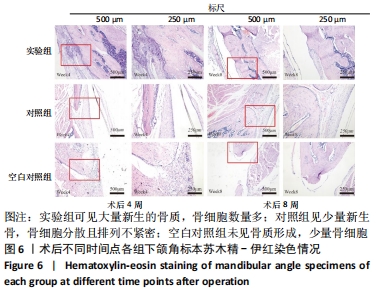
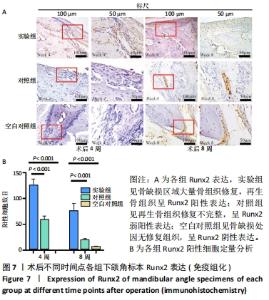
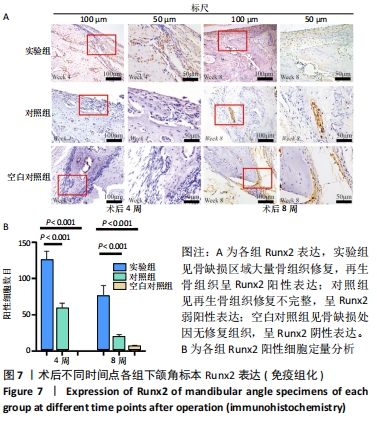
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (28): 4446-4451.doi: 10.12307/2022.296
Previous Articles Next Articles
Electroactive membrane promotes bone formation in rats in vivo
He Yiheng, Cheng Mingwei, Zhu Peijun, Xu Yan, Chen Jiahao, Lai Chunhua, Xu Shulan
- Stomatological Hospital of Southern Medical University, Guangzhou 510000, Guangdong Province, China
-
Received:2021-02-18Accepted:2021-04-15Online:2022-10-08Published:2022-03-18 -
Contact:Xu Shulan, Chief physician, Stomatological Hospital of Southern Medical University, Guangzhou 510000, Guangdong Province, China -
About author:He Yiheng, Master, Physician, Stomatological Hospital of Southern Medical University, Guangzhou 510000, Guangdong Province, China -
Supported by:the Scientific Research Project of Guangdong Provincial Bureau of Traditional Chinese Medicine, No. 20211274 (to XSL); Scientific Research and Cultivation Project of Stomatological Hospital of Southern Medical University, No. PY2020011 (to XSL)
CLC Number:
Cite this article
He Yiheng, Cheng Mingwei, Zhu Peijun, Xu Yan, Chen Jiahao, Lai Chunhua, Xu Shulan. Electroactive membrane promotes bone formation in rats in vivo[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(28): 4446-4451.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] SOLDATOS NK, STYLIANOU P, KOIDOU VP, et al. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017;48(2):131-147. [2] NING C, ZHOU Z, TAN G, et al. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog Polym Sci. 2018; 81:144-162. [3] ZHENG T, HUANG Y, ZHANG X, et al. Mimicking the electrophysiological microenvironment of bone tissue using electroactive materials to promote its regeneration. J Mater Chem B. 2020;8(45):10221-10256. [4] RIBEIRO C, CORREIA DM, RIBEIRO S, et al. Piezoelectric poly(vinylidene fluoride) microstructure and poling state in active tissue engineerin. Eng Life Sci. 2015;15(4):351-356. [5] 熊莹,许燕,周建平,等.组织工程研究中的电活性生物材料[J].中国组织工程研究,2019,23(34):5523-5530. [6] KHARE D, BASU B, DUBEY AK. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials. 2020;258(11):120280. [7] TANDON B, BLAKER JJ, CARTMELL SH. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018;73:1-20. [8] LIUXIA R, XIANNIAN Y, YUFANG C, et al. Properties and Applications of the β Phase Poly(vinylidene fluoride). Polymers. 2018;10(3):228. [9] LI Y, DAI X, BAI Y, et al. Electroactive BaTiO nanoparticle-functionalized fibrous scaffolds enhance osteogenic differentiation of mesenchymal stem cells. Int J Nanomed. 2017;12:4007-4018. [10] PÄRSSINEN J, HAMMARÉN H, RAHIKAINEN R, et al. Enhancement of adhesion and promotion of osteogenic differentiation of human adipose stem cells by poled electroactive poly(vinylidene fluoride). J Biomed Mater Res A. 2015;103(3):919-928. [11] 程鸣威,徐淑兰,赖春花.极化P(VDF-TrFE)生物膜促进骨髓间充质干细胞成骨分化的实验研究[J].中国口腔医学继续教育杂志, 2019,7(Z):103. [12] DAS A, FISHERO BA, CHRISTOPHEL JJ, et al. Poly(lactic-co-glycolide) polymer constructs cross-linked with human BMP-6 and VEGF protein significantly enhance rat mandible defect repair. Cell Tissue Res. 2015; 364(1):125-135. [13] YAMANO S, HAKU K, YAMANAKA T, et al. The effect of a bioactive collagen membrane releasing PDGF or GDF-5 on bone regeneration. Biomaterials. 2014;35(8):2446-2453. [14] KORUPALLI C, LI H, NGUYEN N, et al.Conductive Materials for Healing Wounds: Their Incorporation in Electroactive Wound Dressings, Characterization, and Perspectives. Adv Healthcare Mater. 2021;10: 2001384. [15] 任立志,孙睿.引导骨再生屏障膜材料临床应用进展[J].口腔疾病防治,2020,28(6):404-408. [16] PALZA H, ZAPATA PA, ANGULO-PINEDA C. Electroactive Smart Polymers for Biomedical Applications. Materials. 2019;12(2):277. [17] LEE MK, DECONDE AS, LEE M, et al. Biomimetic scaffolds facilitate healing of critical-sized segmental mandibular defects. Am J Otolaryng. 2015;36(1):1-6. [18] VANESSA C, DANIELA C, CLARISSE R, et al. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers. 2018; 10(2):161. [19] GOPINATHAN J, NOH I. Current Status of Development and Intellectual Properties of Biomimetic Medical Materials. Adv Exp Med Biol. 2018; 1064:377-399. [20] CHEN Y, KIM YS, TILLMAN BW, et al. Advances in Materials for Recent Low-Profile Implantable Bioelectronics. Materials. 2018;11(4):522. [21] PEYRIN F. Evaluation of bone scaffolds by micro-CT. Osteoporosis Int. 2011;22(6):2043-2048. [22] SCALIZE PH, BOMBONATO-PRADO KF, SOUSA LD, et al. Poly(Vinylidene Fluoride-Trifluorethylene)/barium titanate membrane promotes de novo bone formation and may modulate gene expression in osteoporotic rat model. J Mater Sci-mater M. 2016;27(12):1-10. [23] WANG Q, HUANG Y, QIAN Z. Nanostructured Surface Modification to Bone Implants for Bone Regeneration. J Biomed Nanotechnol. 2018; 14(4):628-648. [24] BERTUCCI C, KOPPES R, DUMONT B, et al. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Res Bull. 2019;152:265-284. [25] THRIVIKRAMAN G, BODA SK, BASU B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials. 2018;150: 60-86. [26] JACOB J, MORE N, KALIA K, et al. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regener. 2018;38(1):2. [27] ZHANG C, LIU W, CAO C, et al. Modulating Surface Potential by Controlling the beta Phase Content in Poly(vinylidene fluoridetrifluoroethylene) Membranes Enhances Bone Regeneration. Adv Healthc Mater. 2018;7(11):e1701466. [28] MCLAUGHLIN KA, LEVIN M. Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form. Dev Biol. 2018; 433(2):177. [29] LEVIN M, PEZZULO G, FINKELSTEIN JM. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu Rev Biomed Eng. 2017;19:353-387. [30] KOMORI T. Molecular Mechanism of Runx2-Dependent Bone Development. Mol Cells. 2020;43(2):168-175. [31] KOMORI T. Roles of Runx2 in Skeletal Development. Adv Exp Med Biol. 2017;962:83-93. [32] BAXTER FR, BOWEN CR, TURNER IG, et al. Electrically Active Bioceramics: A Review of Interfacial Responses. Ann Biomed Eng. 2010; 38(6):2079-2092. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [3] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [4] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [5] | Wu Li, Huang Wei, Li Xuetao, Li Panpan, Zhang Kaihang, Shen Zhiyuan. Analysis of adhesion and permeability of bone scaffold with Voronoi architecture [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(28): 4472-4476. |
| [6] | Fan Yi, Liu Yadong, Cui Yutao, Liu He, Tian Yuhang, Li Shaorong, Wang Gan, Wu Dankai, Peng Chuangang. Modification of natural and composite alginate hydrogels and repair of bone defect with composite systems [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(28): 4532-4538. |
| [7] | Yun Xiao, Ding Tong, Yang Weiqiang, Guo Xinjun. Nano hydroxyapatite/chitosan scaffold loaded with Akebia saponin D in bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4293-4299. |
| [8] | Zhao Doudou, Lin Kaili. Application of multicellular construction of vascularized tissue engineered bone in bone repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4386-4392. |
| [9] | Zhong Ruiying, Wang Fuke, Yang Guiran, Wang Guoliang, Hou Jianfei, Liao Xinyu. Co-culture of fibroblasts and vascular endothelial cells affects proliferation and osteogenesis of adipose stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3833-3839. |
| [10] | Lu Haiping, Lang Xuemei, Zhang Cheng, Ju Songli, Zhang Yi, Wang Xin. Application of polycaprolactone modified biological barrier membrane in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3580-3585. |
| [11] | Guo Sutong, Guo Yu, Wang Ling, Li Yaxin, Ding Yujian, Gao Jingfeng, Xu Haitao, Ren Tianhao, Feng Dehong. Application of copper ion in bone tissue engineering: biocompatibility, antibacterial properties, angiogenic activity and osteogenic activity [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(21): 3410-3414. |
| [12] | Tian Yuhang, Liu Yadong, Cui Yutao, Liu He, Li Shaorong, Wang Gan, Fan Yi, Peng Chuangang, Wu Dankai. Application of chitosan biomaterial scaffold in the treatment of infectious bone defects [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(21): 3415-3420. |
| [13] | Wang Diaodiao, Sun Yuyang, Tian Zhuang, Zhang Chu, Li Hanchen, Yao Qi. Relationship of the design of different bone tissue engineering scaffolds with the changes of osteoconduction, osteoinductivity and biodegradability [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(21): 3435-3444. |
| [14] | Wang Weiwei, Ou Zhixue, Zhang Xiaoyun, Li Shibin, Zhou Yi, Li Tong. Regulatory mechanism of exosomes in signal communication network of steroid induced avascular necrosis of the femoral head repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3056-3064. |
| [15] | Li Yaoming, Jiang Hong, Shi Yongfang. Preparation and characterization of chitosan/polylactic acid/hydroxyapatite/polyvinyl alcohol composite bone scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(18): 2888-2893. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||