Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (21): 3410-3414.doi: 10.12307/2022.652
Previous Articles Next Articles
Application of copper ion in bone tissue engineering: biocompatibility, antibacterial properties, angiogenic activity and osteogenic activity
Guo Sutong, Guo Yu, Wang Ling, Li Yaxin, Ding Yujian, Gao Jingfeng, Xu Haitao, Ren Tianhao, Feng Dehong
- Department of Orthopedics, Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi 214000, Jiangsu Province, China
-
Received:2021-03-12Accepted:2021-04-28Online:2022-07-28Published:2022-01-28 -
Contact:Feng Dehong, Chief physician, Associate professor, Department of Orthopedics, Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi 214000, Jiangsu Province, China -
About author:Guo Sutong, Master candidate, Department of Orthopedics, Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi 214000, Jiangsu Province, China -
Supported by:Wuxi Social Development Science and Technology Demonstration Project (Medical and Health) in 2019, No. N20192006 (to FDH)
CLC Number:
Cite this article
Guo Sutong, Guo Yu, Wang Ling, Li Yaxin, Ding Yujian, Gao Jingfeng, Xu Haitao, Ren Tianhao, Feng Dehong. Application of copper ion in bone tissue engineering: biocompatibility, antibacterial properties, angiogenic activity and osteogenic activity[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(21): 3410-3414.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
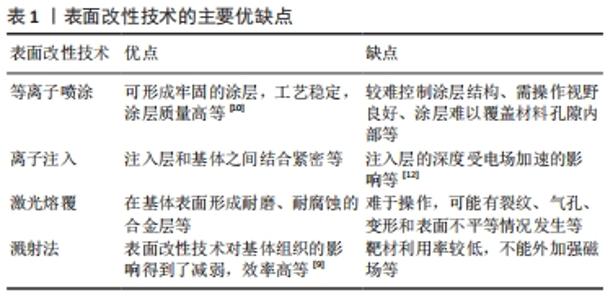
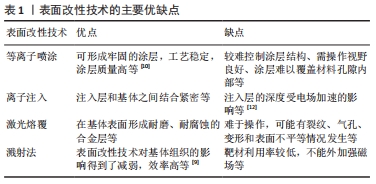
2.1 铜离子表面改性技术 针对铜离子在骨组织工程中所面临的问题,已有多种表面改性技术进行处理。表面改性方法主要有[9-12]:等离子喷涂指将熔融或半熔融状态的涂料高速喷向材料表面,冷却后形成牢固涂层的表面改性技术;离子注入指将离子经电场加速后,以较高的能量射入材料表面获得与基体成分不同的改性层,缺点是注入层的深度受电场加速的影响;激光熔覆技术指利用激光束同时快速熔化涂布在材料表面的熔覆材料与基体表面的部分材料,在基体表面形成耐磨、耐腐蚀的合金层;溅射法指从靶材中溅射出的原子沉积到材料表面,获得成分分布较均匀的薄膜,优点是效率高,溅射过程主要针对靶材,表面改性技术对基体组织的影响得到了减弱。上述当前主要表面改性技术的主要优缺点见表1。"

2.2 铜离子的生物相容性 生物相容性是机体对非活性材料产生的一种本能反应,以表示材料与生物体互相作用的生物学行为。生物相容性主要包括以下两项原则:生物功能性原则和生物安全性原则。生物材料在应用于临床之前必须确保对机体无致敏性、无毒性、无刺激性。在生物相容性范围内,如何设计保证足够多的活性成分长期释放并产生生物活性的表面修饰,是一个极具挑战性的课题。 铜离子是人体含量第二的微量元素,对于心脑血管系统、消化系统、神经系统、骨组织及结缔组织起着十分重要的作用[13]。人体缺乏铜可引起贫血、冠心病、骨质疏松等疾病[14]。铜离子还具有良好的成骨作用和抗关节炎作用[15],铜离子的缺乏导致骨组织强度降低,增加骨性关节炎的发生概率。 验证骨科植入物的生物相容性的方法主要有:皮肤刺激实验、短期全身毒性实验、溶血实验、血浆复钙时间实验、凝血酶原时间实验及动态凝血实验等[16]。LIU 等[17]通过体外细胞培养实验验证了铜钛合金具有良好的细胞相容性。MILKOVIC等[18]观察含不同浓度(质量分数0,0.1,12.5%)铜元素生物玻璃表面上骨细胞的增殖反应,结果提示0.1%的铜无细胞毒性。ZHANG等[16]的实验研究了4种不同铜含量铜钛合金材料对生物相容性的影响,细胞相对生长率测试和CCK-8表明铜钛合金对MG63细胞未见明显毒性。 2.3 铜离子的抗菌效果 漂浮细菌黏附到假体表面形成生物膜是假体周围感染的第一步。生物膜的形成包含表面细菌附着的初始快速阶段、胞外多糖基质中多层细胞增殖阶段和细胞间黏附阶段。细菌可在假体表面黏附、增殖并形成生物膜,黏附的细菌会产生一种胞外物质,使得这些细菌比在体内漂浮的单个细菌更难根除[19]。因此,预防假体周围感染的关键是预防细菌的黏附及增殖。 铜离子具有良好的抗菌效果[11],可有效抑制或杀灭金黄色葡萄球菌和大肠杆菌[20]、肺炎克雷伯菌[21]、结核分枝杆菌[22]、沙门氏菌和空肠弯曲菌等[23]。DU等[24]研究铜和银对抗耐甲氧西林金黄色葡萄球菌疗效,发现C11000铜的抗菌效果明显优于银合金、S30400不锈钢等材料,故其建议铜元素为复合型生物材料中抗菌元素的首选。LIU等[17]的研究结果表明,铜钛合金对于金黄色葡萄球菌和大肠埃希菌具有较强的抗菌性,在含铜量由低到高的合金表面,抗菌作用随着培养时间增强。YOUSEF 等[21]使用铜纳米颗粒纤维对肺炎克雷伯菌的抗菌活性进行了测试,结果显示随着铜含量的增加抗菌作用增强。研究表明,细菌细胞壁损伤和DNA凝聚沉积明显,结果显示漂浮金黄色葡萄球菌的生长受阻,附着于材料表面的细菌可在24 h内被清除[25]。 然而,关于铜离子的抑菌浓度问题一直存在争议。DU等[24]的研究证实,铜离子对金黄色葡萄球菌和大肠杆菌最小抑菌浓度分别为448,256 mg/L。ZHANG等[16]的研究表明,在1%和5%铜钛合金中随着铜含量的增加,合金对金黄色葡萄球菌和大肠杆菌的抗菌作用及对成纤维细胞的细胞毒性作用增强;1%的铜含量在限制细菌感染和保证组织整合方面是最有希望的,10%铜钛合金未观察到抑菌晕,但10%铜钛合金及1%和5%铜钛合金均观察到黏附在表面的细菌显著减少;在10%铜钛合金中,较低的铜离子释放(在生理溶液中72 h后释放0.05 mg/L)表明,细菌只有在直接接触合金表面时才能被杀死。LI等[26]制备了不同铜含量(质量分数0.05,0.1,0.25%)的可生物降解镁铜合金材料,研究发现其不仅具有优良的抗菌效果,还可抑制生物膜和抗生素抵抗基因的表达,结果显示含0.25%铜的实验组具有最好的抗菌活性。 当前研究认为铜离子的抗菌作用机制主要有[27-30]:铜离子能与巯基结合,从而改变细菌细胞膜的通透性,产生活性氧,使细菌蛋白质氧化和DNA的降解,导致细菌死亡;铜离子可干扰细胞分裂和复制进而影响细菌DNA的合成;铜离子可改变细菌细胞壁中蛋白质的结构和功能,引起细胞破裂。 2.4 铜离子的促血管生成活性 血管生成被认为是组织修复的生物学基础。从细胞层面说,血管生成包括以下几个阶段[31]:内皮细胞前体细胞分化为内皮细胞;分化的内皮细胞聚集,细胞间各自黏附建立连接;连接的内皮产生管腔;新生内皮细胞管腔凝集形成原始血管网;平滑肌、细胞基质及血管外皮细胞长入形成血管。 铜离子与成纤维细胞生长因子有密切联系,它们对血管生成具有协同刺激效应。铜离子可直接促进血管内皮生长因子的产生[32-33]。血管内皮生长因子通过调节周围组织中的内皮细胞的增殖、迁移,最终形成管状结构,从而激活内皮细胞[34-35]。铜离子还可通过提高缺氧诱导因子1α的表达、抑制缺氧诱导因子1α的降解来促进内皮细胞的募集、分化和血管生成[36-37],同时缺氧诱导因子1α的活性又对血管内皮生长因子表达的转录有促进作用[38]。缺氧诱导因子靶点肾上腺髓质素被证明可以促进生理和病理血管生成,肾上腺髓质素通过结合降钙素受体介导血管生成,广泛表达于正常和缺氧的内皮细胞上[39]。LI等[40]的研究表明,含铜生物活性玻璃/蛋壳膜纳米复合材料可提高内皮型一氧化氮合酶基因表达。内皮型一氧化氮合酶在体内主要负责产生一氧化氮,一氧化氮对细胞生长的作用是调节细胞周期的进程,高浓度一氧化氮促进内皮细胞G1期向S期转化,低浓度一氧化氮促进G2期向M期转化。有研究表明,ATP7A铜转运体通过促进细胞外基质重构和抗氧化铜原酶的分泌,并维持Rac信号传导,积极维持生长因子依赖的血管重构[41-43]。在此过程中,ATP7A泵与铜特异性转运蛋白一起工作,为血管细胞提供足够的铜[44-45]。 2.5 铜离子的成骨活性 无菌性松动和内植物感染似乎是相互排斥的,然而这两个问题是又密切相关的。若宿主细胞抢先占领内植物表面,这不仅会增强组织的整合,也将阻止微生物的黏附和增殖[46]。虽然各种各样的研究提供了改善骨整合的策略和预防感染策略,但通常建议的解决方案只解决了两个问题中的一个。促进骨整合和预防内植物感染是理想的骨科内植物必须同时具备的2个要求。 无菌性松动的原因主要是内植物和组织界面间的微动。随着内植物的使用,小的微动会进展为大的微动,最终导致植入的失败。骨和植入物间的间隙也可因植入物的磨屑导致无菌性松动[47],磨损颗粒沿着内植物长轴流动间隙构成的管道,抑制假体与骨的接触。此外,巨噬细胞不易吞噬磨损颗粒,激活细胞炎症反应并分泌一系列的细胞因子,这些细胞因子可导致骨组织的吸收和破骨细胞的产生,形成并扩大假体与骨组织间的间隙,进而发展为严重松动,最终导致手术失败。 研究发现,骨科植入物与宿主的结合分机械结合、生物结合及骨键合3种形态[48]。骨整合,即金属假体与骨通过化学键成为一整体,具备生物力学传导均匀性和直接性,键合的强度甚至超出了假体或骨本身,使假体获得永久的稳定性[49-50]。良好的植入生物材料需具备两种生物活性:促进骨整合及抑制破骨细胞的活性。如何获得良好的骨键合,己经成为评价人工假体生物活性及疗效的一项重要指标。 BURGHARDT等[51]设计了不同浓度的铜钛合金,发现铜离子在0.1 mmol/L浓度范围可促进间充质干细胞的增殖、成骨分化,促进碱性磷酸酶活性增加、Ⅰ型胶原、骨保护素、骨桥蛋白基因表达和细胞矿化。EWALD等[52]在体外研究了铜离子对刷状支架和玻璃盘上成骨细胞生长和活性的影响,结果表明铜离子增强了细胞活性和成骨细胞的增殖,并影响骨涎蛋白、骨钙素等多种骨特异性蛋白的表达,以刺激细胞活性,改善骨愈合。HUANG等 [28]制备了氧化石墨烯-铜纳米复合材料,实验结果表明,氧化石墨烯-铜纳米复合材料涂层能增强大鼠骨髓间充质干细胞的黏附和成骨能力,氧化石墨烯-铜纳米复合材料能激活大鼠骨髓间充质干细胞的缺氧诱导因子1α通路,还能通过细胞外信号调节激酶信号传导途径,进一步增强血管内皮生长因子和骨形态发生蛋白2 的表达,磷酸钙/氧化石墨烯-铜纳米复合材料支架能显著促进大鼠颅骨缺损处的骨再生。LI等[53]将铜掺杂在聚磷酸钙支架上,该支架与低氧预处理的骨髓间充质干细胞联合使用可以上调缺氧诱导因子1α、血管内皮生长因子、碱性磷酸酶和骨钙素的水平,从而更好地促进新骨形成。WU等[38]制备了含铜的介孔生物活性玻璃支架,其离子提取物均可激活人骨髓间充质干细胞的缺氧诱导因子1α 通路,可以通过提高人骨髓间充质干细胞成骨相关基因(如碱性磷酸酶、骨桥蛋白、骨钙素等)的表达来促进人骨髓间充质干细胞的成骨分化,含铜介孔生物活性玻璃支架可明显促进骨再生。骨髓间充质干细胞对铜的正常摄取通常由铜三聚体转运蛋白介导,当骨髓间充质干细胞摄取铜后,铜在细胞发挥相应的作用;在细胞中缺氧诱导因子1α与缺氧反应元件序列的结合过程中,铜可能是必须的元素,但是该过程是依赖超氧化歧化酶。 骨免疫是当前提高假体骨结合的研究热点[54]。假体植入会引起宿主的免疫反应,因此直接调控骨髓间充质干细胞成骨分化虽可取得成效,但仍有部分体外效应与体内效应有差异。受不同微环境影响,巨噬细胞可极化为促炎型M1和损伤修复型M2[55]。M1型可分泌肿瘤坏死因子α、白细胞介素6、白细胞介素1β等促炎因子,诱导破骨细胞生成和增殖,导致骨吸收。M2型是白细胞介素6、白细胞介素10等抗炎因子的主要来源,其分泌的血管内皮生长因子、转化生长因子β、骨形态生成蛋白2等细胞因子可促进血管生成和基质重塑[56-57]。高效而适时的M1型极化可使M2型释放成骨增强因子,并促进新骨形成[58]。当前研究结果表明,铜离子可通过激活巨噬细胞中的Cu转运信号(如铜转运蛋白1和ATP7A),使巨噬细胞极化为促炎性M1表型[48, 59];通过调节整联蛋白(α5,αM,β1和β2)和Toll样受体(TLR-3,TLR-4,Myd88和Ticam-1/2)信号传导在一定程度上显示出抗炎作用。生长在铜钛合金表面或经Cu2 +处理的巨噬细胞可为成骨细胞样SaOS-2细胞的增殖和分化提供炎症微环境。实验结果表明,铜钛合金表面可致骨整合和M1表面标志物CD11c、生长因子骨形态发生蛋白6及成骨细胞包括骨钙蛋白和Runx-2的表达水平提高。 但是,关于铜离子适宜成骨浓度也有待进一步讨论。CHAI等[60]设计了含铜317L不锈钢预防植入物相关感染的抗菌效果体内外实验,观察铜对生物体内成骨细胞功能的反应,发现含4.5%铜的317L-Cu兼具抗菌活性、骨诱导性和骨整合性等多种生物活性。MILKOVIC等[18]设计了掺杂铜元素生物玻璃骨细胞脂质过氧化诱导实验,观察含不同浓度(质量分数0,0.1%,12.5%)铜元素的生物玻璃表面上骨细胞增殖反应,结果提示0.1%的铜可增强脂质过氧化反应并促进骨细胞生长,无细胞毒性;而含12.5%铜的生物玻璃表面的骨细胞生长则受到明显抑制。较低浓度的铜离子可促进骨髓间充质干细胞的成骨分化,而高浓度的铜离子则具有优良的抗菌作用[51]。 综上述可知,在细胞水平上,铜离子可促进成骨细胞分化、抑制破骨细胞分化,进而影响骨的代谢;在分子生物学水平上,铜离子可通过影响缺氧诱导因子1α通路、ERK1/2信号传导、整联蛋白信号传导、Toll样受体信号传导等,从而影响骨的代谢。"

| [1] DAIGLE ME, WEINSTEIN AM, KATZ JN, et al. The cost-effectiveness of total joint arthroplasty: a systematic review of published literature. Best Pract Res Clin Rheumatol. 2012;26(5):649-658. [2] MCGRORY BJ, ETKIN CD, LEWALLEN DG. Comparing contemporary revision burden among hip and knee joint replacement registries. Arthroplast Today. 2016;2(2):83-86. [3] BOZIC KJ, KURTZ SM, LAU E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51. [4] ZMISTOWSKI B, KARAM JA, DURINKA JB, et al. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013;95(24): 2177-2184. [5] SENDI P, FREI R, MAURER TB, et al. Escherichia coli variants in periprosthetic joint infection: diagnostic challenges with sessile bacteria and sonication. J Clin Microbiol. 2010;48(5):1720-1725. [6] NURYASTUTI T, KROM BP, AMAN AT, et al. Ica-expression and gentamicin susceptibility of Staphylococcus epidermidis biofilm on orthopedic implant biomaterials. J Biomed Mater Res A. 2011;96(2):365-371. [7] SHIRAI T, TSUCHIYA H, SHIMIZU T, et al. Prevention of pin tract infection with titanium-copper alloys. J Biomed Mater Res B Appl Biomater. 2009; 91(1):373-380. [8] HEIDENAU F, MITTELMEIER W, DETSCH R, et al. A novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization. J Mater Sci Mater Med. 2005;16(10):883-888. [9] 董运涛,樊科社,吴江涛,等.磁控溅射-熔覆法制备铜/钼接头的组织与性能研究[J].热加工工艺,2020,49(20):72-74+79. [10] 郇庆婷,刘雅琴,蔡宏章,等.等离子喷涂Cu-Al_2O_3复合涂层的制备及性能研究[J].热加工工艺,2020,49(20):88-91. [11] DUAN J, YANG Y, ZHANG E, et al. Co-Cr-Mo-Cu alloys for clinical implants with osteogenic effect by increasing bone induction, formation and development in a rabbit model. Burns Trauma. 2020;8:tkaa036. [12] 李昆强,乔玉琴,刘宣勇.钛表面铜离子注入对细菌和细胞行为的影响[J].无机材料学报,2020,35(2):158-164. [13] SHIM H, HARRIS ZL. Genetic defects in copper metabolism. J Nutr. 2003; 133(5 Suppl 1):1527s-1531s. [14] WAPNIR RA. Copper absorption and bioavailability. Am J Clin Nutr. 1998; 67(5 Suppl):1054s-1060s. [15] ZHANG E, LIU C. A new antibacterial Co-Cr-Mo-Cu alloy: Preparation, biocorrosion, mechanical and antibacterial property. Mater Sci Eng C Mater Biol Appl. 2016;69:134-143. [16] ZHANG E, ZHENG L, LIU J, et al. Influence of Cu content on the cell biocompatibility of Ti-Cu sintered alloys. Mater Sci Eng C Mater Biol Appl. 2015;46:148-157. [17] LIU J, LI F, LIU C, et al. Effect of Cu content on the antibacterial activity of titanium-copper sintered alloys. Mater Sci Eng C Mater Biol Appl. 2014;35: 392-400. [18] MILKOVIC L, HOPPE A, DETSCH R, Et al. Effects of Cu-doped 45S5 bioactive glass on the lipid peroxidation-associated growth of human osteoblast-like cells in vitro. J Biomed Mater Res A. 2014;102(10):3556-3561. [19] WANG R, NEOH KG, SHI Z, et al. Inhibition of Escherichia coli and Proteus mirabilis adhesion and biofilm formation on medical grade silicone surface. Biotechnol Bioeng. 2012;109(2):336-354. [20] VILLANUEVA ME, DIEZ AM, GONZÁLEZ JA, et al. Antimicrobial Activity of Starch Hydrogel Incorporated with Copper Nanoparticles. ACS Appl Mater Interfaces. 2016;8(25):16280-16288. [21] YOUSEF A, BARAKAT NAM, AMNA T, et al. Inactivation of pathogenic Klebsiella pneumoniae by CuO/TiO2 nanofibers: A multifunctional nanomaterial via one-step electrospinning. Ceram Int. 2012;38(6):4525-4532. [22] DALECKI AG, HAEILI M, SHAH S, et al. Disulfiram and Copper Ions Kill Mycobacterium tuberculosis in a Synergistic Manner. Antimicrob Agents Chemother. 2015;59(8):4835-4844. [23] FAÚNDEZ G, TRONCOSO M, NAVARRETE P, et al. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol.2004;4:19. [24] DU WL, NIU SS, XU YL, et al. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym. 2008;75(3):385-389. [25] HASSAN MS, AMNA T, KIM HY, et al. Enhanced bactericidal effect of novel CuO/TiO 2 composite nanorods and a mechanism thereof. Composites Part B. 2013;45(1):904-910. [26] LI Y, LIU L, WAN P, et al. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations. Biomaterials. 2016;106:250-263. [27] LEMIRE JA, HARRISON JJ,TURNER RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013; 11(6):371-384. [28] HUANG L, FOZO EM, ZHANG T, et al. Antimicrobial behavior of Cu-bearing Zr-based bulk metallic glasses. Mater Sci Eng C Mater Biol Appl. 2014;39: 325-329. [29] CHATTERJEE AK, CHAKRABORTY R, BASU T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 2014;25(13):135101. [30] GHIMIRE N, FOSS BL, SUN Y, et al. Interactions among osteoblastic cells, Staphylococcus aureus, and chitosan-immobilized titanium implants in a postoperative coculture system: An in vitro study. J Biomed Mater Res A. 2016;104(3):586-594. [31] GERHARDT H, BETSHOLTZ C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314(1):15-23. [32] ZHOU Y, JIANG Y, KANG YJ. Copper inhibition of hydrogen peroxide-induced hypertrophy in embryonic rat cardiac H9c2 cells. Exp Biol Med (Maywood). 2007;232(3):385-389. [33] ZHOU Y, JIANG Y, KANG YJ. Copper reverses cardiomyocyte hypertrophy through vascular endothelial growth factor-mediated reduction in the cell size. J Mol Cell Cardiol. 2008;45(1):106-117. [34] SHO E, KOMATSU M, SHO M, et al. High flow drives vascular endothelial cell proliferation during flow-induced arterial remodeling associated with the expression of vascular endothelial growth factor.Exp Mol Pathol. 2003; 75(1):l1-11. [35] JOZKOWICZ A, BROSTJAN C, NIGISCH A, et al. Effects of 15d-PGJ2 on VEGF-induced angiogenic activities and expression of VEGF receptors in endothelial cells. Vas Pharmacol. 2006;45(3):e20-e21. [36] MARTIN F, LINDEN T, KATSCHINSKI DM, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood. 2005;105(12):4613-4619. [37] QIN L, HAOBIN C, XI H, et al. Effects of 12 metal ions on iron regulatory protein 1 (IRP-1) and hypoxia-inducible factor-1 alpha (HIF-1alpha) and HIF-regulated genes. Toxicol Appl Pharmacol. 2006;213(3):245-255. [38] WU C, ZHOU Y, XU M, et al. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34(2): 422-433. [39] SHINDO T, KURIHARA Y, NISHIMATSU H, et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104(16):1964-1971. [40] LI J, ZHAI D, LV F, et al. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016;36:254-266. [41] ASHINO T, SUDHAHAR V, URAO N, et al. Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res. 2010;107(6):787-799. [42] CARINE W, JAEKWON L, TAIHO K, et al. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009; 284(49):33949-33956. [43] ZHAN H, LIANG H, LIU X, et al. Expression of Rac1, HIF-1α, and VEGF in Gastric Carcinoma: Correlation with Angiogenesis and Prognosis. Onkologie. 2013;36(3):102-107. [44] PETRIS MJ, SMITH K, LEE J, et al. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003; 278(11):9639-9646. [45] Petris MJ, Smith K, Lee J, et al. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem biological chemistry. 2003;278(11):9639-9646. [46] GREENFIELD EM, BI Y, RAGAB AA, et al. Does endotoxin contribute to aseptic loosening of orthopedic implants? J Biomed Mater Res B Appl Biomater. 2005;72(1):179-185. [47] CHERIAN JJ, JAUREGUI JJ, BANERJEE S, et al. What Host Factors Affect Aseptic Loosening After THA and TKA? Clin Orthop Relat Res. 2015;473(8): 2700-2709. [48] HUANG Q, OUYANG Z, TAN Y, et al. Activating macrophages for enhanced osteogenic and bactericidal performance by Cu ion release from micro/nano-topographical coating on a titanium substrate .Acta Biomater. 2019; 100:415-426. [49] LAPPALAINEN R, SANTAVIRTA SS. Potential of coatings in total hip replacement. Clin Orthop Relat Res. 2005;(430):72-79. [50] KIM TI, JANG JH, KIM HW, et al. Biomimetic approach to dental implants. Curr Pharm Des. 2008;14(22):2201-2211. [51] BURGHARDT I, LÜTHEN F, PRINZ C, et al. A dual function of copper in designing regenerative implants. Biomaterials. 2015;44:36-44. [52] EWALD A, KÄPPEL C, VORNDRAN E, et al. The effect of Cu(II)‐loaded brushite scaffolds on growth and activity of osteoblastic cells. J Biomed Mater Res A. 2012;100(9):2392-400. [53] LI Y, WANG J, WANG Y, et al. Transplantation of copper-doped calcium polyphosphate scaffolds combined with copper (II) preconditioned bone marrow mesenchymal stem cells for bone defect repair. J Biomater Appl. 2018;32(6):738-753. [54] ARNO MC, WILLIAMS RJ, BEXIS P, et al. Exploiting topology-directed nanoparticle disassembly for triggered drug delivery. Biomaterials. 2018; 180:184-192. [55] DAS A, SINHA M, DATTA S, et al. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am J Pathol. 2015;185(10):2596-606. [56] CHEN Z, BACHHUKA A, WEI F, et al. Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale. 2017;9(46):18129-18152. [57] WU XQ, DAI Y, YANG Y, et al. Emerging role of microRNAs in regulating macrophage activation and polarization in immune response and inflammation. Immunology. 2016;148(3):237-248. [58] Karimpour MA, Esmailnejad MA, Musavi DS. Comparison of conditioned medium and direct co-culture of human granulosa cells on mouse embryo development. Indian J Exp Biol. 2006;44(3):189-192. [59] SHI M, CHEN Z, FARNAGHI S, et al. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016;30:334-344. [60] CHAI H, GUO L, WANG X, et al. Antibacterial effect of 317L stainless steel contained copper in prevention of implant-related infection in vitro and in vivo. J Mater Sci Mater Med. 2011;22(11):2525-2535. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhou Qian, Zhang Qiang, Chen Qiu. Human salivary components and osteoporosis/osteopenia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1439-1444. |
| [3] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [4] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [5] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [6] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277. |
| [7] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [8] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [9] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1107-1112. |
| [10] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [11] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [12] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [13] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [14] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [15] | Xu Lei, Han Xiaoqiang, Zhang Jintao, Sun Haibiao. Hyaluronic acid around articular chondrocytes: production, transformation and function characteristics [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 768-773. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||