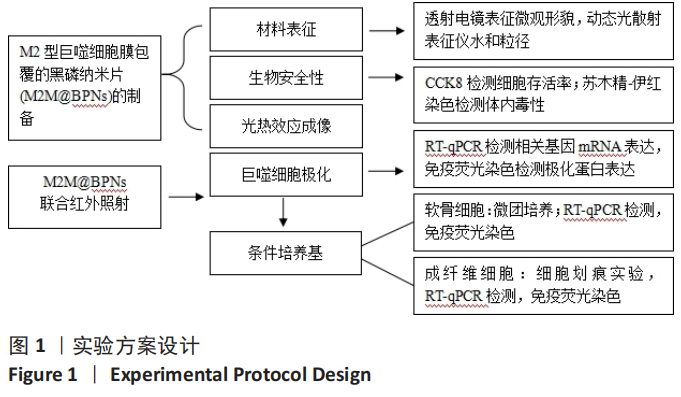
1.1 设计 材料的合成与表征,体外细胞学实验,体内动物实验。 实验方案设计见图1。
1.2 时间及地点 实验于2023年9月至2025年8月在苏州大学附属第一医院骨科研究所完成。
1.3 材料
1.3.1 主要试剂 黑磷(先锋纳米);Ⅱ型胶原酶、胰酶-EDTA、番红O-固绿、TRIzol、DAPI(Sigma-Aldrich,美国);胎牛血清、反转录试剂盒(R323-01)、qPCR检测试剂盒(Q312-02)(诺唯赞公司,中国);DMEM/F12培养基(Keygen BioTECH,中国);α-MEM培养基(凯基生物,中国);青霉素-链霉素(GIBCO,美国);PBS(RG-CE-10,中国Ketu);苏木精-伊红染液(南京建成生物工程研究所,中国);FITC Phalloidin、甲苯胺蓝染液、阿利新蓝染色试剂盒(索莱宝公司,中国);CCK-8试剂盒(C6005)、40 g/L
多聚甲醛(G1101)(赛维尔生物公司,中国);Fluor488标记羊抗兔IgG抗体(S0018,Affinity,中国);Fluor594标记羊抗兔IgG抗体(AS039)、CD86兔单克隆抗体(A21198)、CD206兔多克隆抗体(A8301)、基质金属蛋白酶13兔多克隆抗体(A11755)、Ⅰ型胶原蛋白兔单克隆抗体(A21059)(爱博泰克公司,中国);干扰素γ、脂多糖、白细胞介素4(近岸蛋白,中国);巨噬细胞集落刺激因子(CB34,Novoprotein,中国)。
1.3.2 主要仪器 脂质体挤出器(Avanti,USA);透射电子显微镜(Hitachi,日本);动态光散射仪(DLS,Malvern Zetasizer Nano ZS,德国);红外热成像系统(FOTRIC 616C,中国);实时荧光定量PCR系统(CFX96TM Real-Time PCR system,Bio-Rad,美国);持续吸入式气体麻醉系统(RWD-R500,中国);光学显微镜(Leica,
德国);倒置荧光显微镜(Zeiss Axiovert,德国);NanoDrop
ND-2000分光光度计(Thermo Fisher Scientific,美国);全波长酶标仪(BioTek,美国);CO2细胞培养箱、生物超净台(Thermo Fisher Scienttffc,美国);切片机(Leica RM2165,德国)。
1.3.3 实验细胞 小鼠胚胎成纤维细胞(原代细胞),购自上海硕程生物科技有限公司,采用0.25%胰蛋白酶-0.02% EDTA消化
1 min后重悬于含体积分数10%胎牛血清的DMEM培养基中。计数后,将细胞以每盘5×105的数量铺板,置于在37 ℃、体积分数5%CO2培养箱中。每2 d更换培养基,每三四天传代一次细胞,取P3-P5代成纤维细胞用于实验。
1.3.4 实验动物 8周龄雄性C57BL/6J小鼠34只,1周龄雄性C57BL/6J小鼠8只,均购自杭州启真动物科技有限公司,许可证号:SCXK(浙)2022-0005。所有动物饲喂标准化饲料,置于恒温恒湿、无特定病原体的环境中自由取水,维持12 h明暗交替的昼夜周期。动物实验方案已获苏州大学伦理委员会批准(批准号:SUDA20250320A01。实验过程严格遵循国际兽医学编辑协会《动物伦理与福利作者指南共识》及相关地方和国家法规。在所有手术操作中,实验动物均在麻醉状态下进行,并尽力降低疼痛、应激。
1.4 实验方法
1.4.1 巨噬细胞与软骨细胞的分离培养
巨噬细胞的分离培养:参考文献[26]中的方法分离提取骨髓巨噬细胞。取4只8周龄C57BL/6J小鼠,
经持续吸入式气体麻醉系统麻醉(异氟烷4%,氧浓度30%)后脱颈处死,经体积分数75%乙醇灭菌后转移到无菌玻璃板上,无菌解剖股骨和胫骨,剥去软组织后置于冰冷PBS中,暴露骨髓腔,使用装有生理盐水的20 mL注射器反复冲洗骨髓腔,收集骨髓冲洗液。将骨髓冲洗液离心(500×g离心5 min,4 ℃)后弃上清,用ACK缓冲液裂解红细胞;第二次离心(500×g离心
5 min,4 ℃)后弃上清,将有核细胞重悬于α-MEM培养基中,添加体积分数10%胎牛血清和30 ng/mL巨噬细胞集落刺激因子后接种在10 cm培养皿中。16 h后去除非黏附细胞,培养黏附细胞3 d即获得成熟的骨髓巨噬细胞。
软骨细胞的分离培养:参考文献[27]的方法分离提取软骨细胞。取8只1周龄C57BL/6J小鼠,经持续吸入式气体麻醉系统麻醉(异氟烷4%,氧浓度30%)后脱颈处死,经体积分数75%乙醇灭菌后无菌条件下切取关节软骨,切碎后用10%青霉素-链霉素溶液洗涤,无菌PBS冲洗;在37 ℃下加入0.2%Ⅱ型胶原酶消化过夜,通过70 µm细胞过滤器过滤去除未消化组织,离心(500×g离心5 min)后弃上清,将细胞沉淀重悬于含体积分数10%胎牛血清、100 U/mL青霉素及100 µg/mL 链霉素的DMEM/F-12培养基中,接种于10 cm培养皿中,置于37 ℃、体积分数5% CO₂恒温培养箱中孵育。选择P1代软骨细胞用于后续实验。
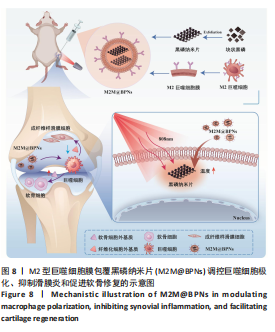
1.4.2 M2型巨噬细胞膜包覆黑磷纳米片的制备 通过改良液相剥离法合成黑磷纳米片(记为BPNs)[28]。将5 mg块体黑磷分散于20 mL去离子水中,并通入氩气以去除溶解氧,随后在冰水浴条件下以探针式超声处理8 h(功率700 W,开/关循环2 s/4 s),所得分散液经3 000 r/min离心20 min移去沉淀,收集呈棕色的上清液于4 ℃保存。使用前以10 000 r/min离心10 min后弃上清,收集棕色沉淀。
将骨髓巨噬细胞在含体积分数10%胎牛血清、20 ng/mL白细胞介素4的DMEM培养基中培养24 h,诱导细胞向M2型极化[29-30];用预冷的裂解缓冲液(50 mmol/L Tris-HCl,pH=7.4;150 mmol/L NaCl;1 mmol/L PMSF;1×蛋白酶抑制剂混合物)裂解并于4 ℃孵育2 h,随后进行3次冻融循环(液氮冷冻
10 min,37 ℃水浴解冻≤5 min);裂解液经500×g离心10 min后收集上清液,12 000×g离心15 min后弃上清,将沉淀重悬于TM缓冲液中作为细胞膜组分,BCA法进行蛋白定量分析(每毫升TM缓冲液中细胞膜蛋白质含量为0.3 mg)。将M2型巨噬细胞细胞膜与BPNs混合(每10 μg BPNs添加0.03 mg M2型巨噬细胞细胞膜),经浴式超声分散15 min,通过脂质体挤出器依次挤过400 nm与200 nm聚碳酸酯膜(各15次),得到M2型巨噬细胞膜包覆黑磷纳米片(记为M2M@BPNs)。
1.4.3 M2M@BPNS的表征
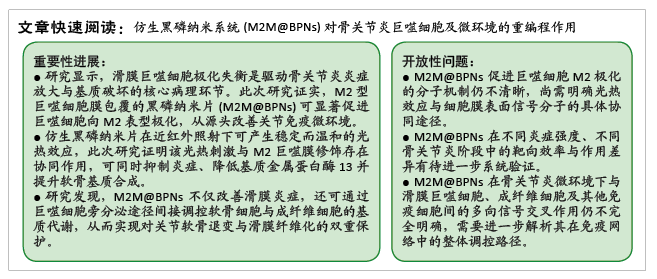
形貌表征:将少量BPNs或M2M@BPNS溶液滴加于铜网载玻片上,轻轻吸附并均匀分布于表面后,用高纯氮气吹干以避免污染,置入透射电子显微镜,根据实验需求选择适当的加速电压(80–200 kV),并在必要时切换至高分辨率模式以观察内部结构与精细形貌。进一步地,将BPNs和M2M@BPNs分别分散于去离子水中,确保体系均匀后置入动态光散射仪,记录颗粒随时间的光散射强度变化,根据结果绘制粒径分布曲线,计算流体动力学粒径。
光热性能评估:将固体BPNs或M2M@BPNS均匀分散于PBS中以避免沉降或团聚,以PBS为对照,使用近红外激光器(808 nm)作为光源,在0.75 W·cm⁻²条件下连续照射3 min,利用红外热成像系统实时监测并记录溶液温度,每30 s获取一次热像图以直观呈现升温过程,绘制温度-时间曲线,分析样品的光热转换效率。
光热循环稳定性:将稳定分散于PBS中的M2M@BPNs置于同一光照条件下(808 nm、0.75 W·cm⁻²),进行连续的加热-冷却循环测试。每个循环中先照射3 min记录温度上升情况,随后自然冷却至室温,重复上述过程3次。整个循环过程中利用红外热成像仪实时监测温度变化,每30 s获取一次热像图以直观呈现温度变化过程。
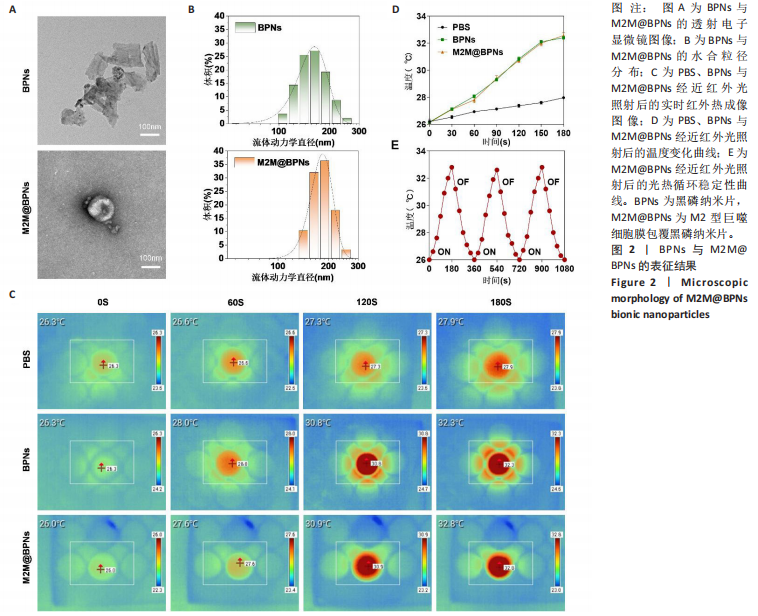
1.4.4 M2M@BPNs的体外细胞相容性评价 将骨髓巨噬细胞以5×103/孔的密度接种于96孔板中,分5组培养:对照组不进行任何处理;脂多糖组加入30 ng/mL巨噬细胞集落刺激因子刺激72 h[22],然后加入100 ng/mL脂多糖和干20 ng/mL扰素γ共同刺激24 h以诱导细胞向M1型极化[29],再加入PBS干预24 h;BPNs组和M2M@BPNs组在诱导细胞向M1型极化后分别加入100 µg/mL的BPNs、M2M@BPNs干预24 h[31-32];M2M@BPNs+红外干预组在诱导细胞向M1型极化后加入100 µg/mL M2M@BPNs干预24 h,期间进行近红外光照射(808 nm,0.75 W/cm²,180 s),每组6复孔。干预结束后,继续培养0,12,24 h,弃去培养液,每孔加入含10 µL CCK-8试剂的100 µL新鲜培养基继续孵育2 h,加入100 µL终止液于25 ℃避光条件下静置4 h,使用酶标仪在570 nm波长下测定吸光度(A)值,计算细胞存活率。以仅加入培养基的孔为空白孔(无细胞无药物)。
细胞存活率(%) = (实验组A值−空白孔A值) / (对照组A值−空白孔A值) × 100%
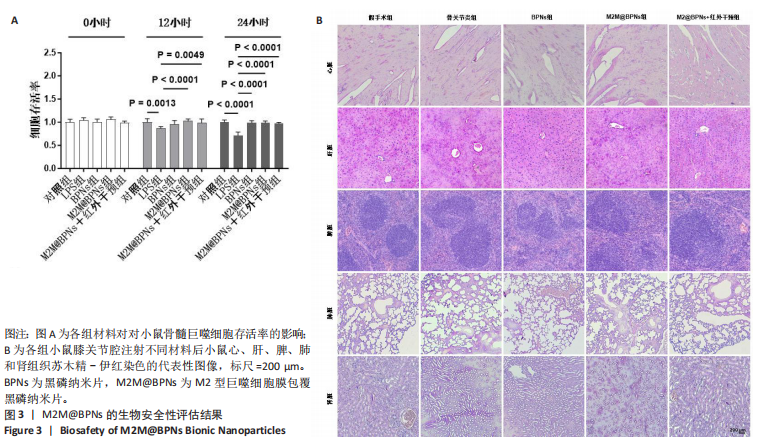
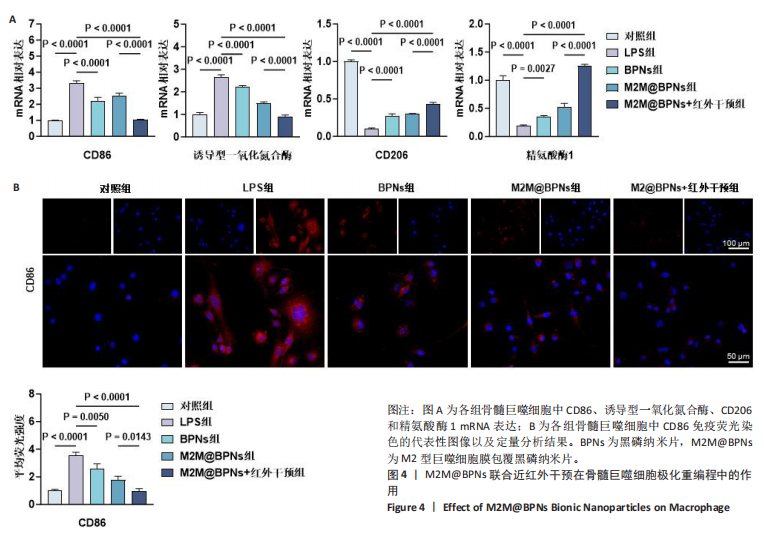
1.4.5 M2M@BPNs联合近红外干预在巨噬细胞极化重编程中的作用 将骨髓巨噬细胞以1×10⁶/孔的密度接种于6孔板中,分对照组、脂多糖组、BPNs组、M2M@BPNs组和M2M@BPNs+近红外干预组,干预方式同1.4.4。干预结束后,RT-qPCR检测CD86、诱导型一氧化氮合酶、CD206、精氨酸酶1 mRNA表达,免疫荧光染色检测CD86表达。
1.4.6 M2M@BPNs联合近红外干预对骨关节炎软骨细胞基质稳态的间接调控 干预结束后,取1.4.5中5组骨髓巨噬细胞培养上清,在4 ℃下1 000×g离心5 min后取上清,分别与DMEM/F-12培养基以1∶1体积比混合,随后添加体积分数10%胎牛血清和1%青霉素-链霉素,获得巨噬细胞条件培养基。
将软骨细胞以1×10⁶ /孔密度接种于6孔板中,待细胞贴壁后分组干预,对照组加入2 mL α-MEM培养基,其余4组分别加入上述4组巨噬细胞条件培养基2 mL。培养24 h后,RT-qPCR检测聚集蛋白聚糖、Ⅱ型胶原蛋白、基质金属蛋蛋白酶13和解整合素-金属蛋白酶5 mRNA表达,免疫荧光染色检测基质金属蛋白酶13表达,分析M2M@BPNs联合近红外干预对软骨细胞基质稳态的间接调控作用。
将软骨细胞以2×1010 L-1的浓度悬浮于含体积分数10%胎牛血清和1%青霉素-链霉素的DMEM/F-12培养基(完全培养基)中。取50 μL软骨细胞悬液滴加于24孔板孔中央,置于37 ℃、体积分数5%CO₂培养箱中培养2 h,观察细胞贴壁后分组培养,对照组加入1 mL完全培养基,其余4组分别加入4组巨噬细胞条件培养基1 mL,每24 h更换培养基。培养72 h后,将形成的软骨细胞微团球在4 ℃条件下用40 g/L多聚甲醛固定24 h,分别进行阿尔新蓝(pH=0.2)与番红O-固绿染色,观察硫酸化糖胺聚糖沉积情况,使用Image J软件定量分析细胞外基质沉积量。
1.4.7 M2M@BPNs联合近红外干预对骨关节炎成纤维细胞代谢稳态的间接调控 将成纤维细胞以1×10⁶/孔的密度接种于6孔板中,分组干预,对照组加入2 mL常规完全培养基,其余4组分别加入4组巨噬细胞条件培养基2 mL。培养24 h后,RT-qPCR检测Ⅰ型胶原蛋白、Ⅱ型胶原蛋白、α-平滑肌肌动蛋白mRNA表达,免疫荧光染色检测Ⅰ型胶原蛋白表达。
细胞划痕实验:将成纤维细胞以1×10⁶/孔的密度接种于6孔板中,分组干预,对照组加入2 mL常规完全培养基,其余4组分别加入4组巨噬细胞条件培养基2 mL。观察细胞形成致密单层后,使用无菌200 μL移液枪头垂直于板面轻轻划出标准化线性划痕,操作过程中保持压力和角度一致,以尽量减少实验差异。划痕完成后,每孔用预热PBS清洗2次以去除脱落细胞和碎屑,将原培养基更换为等体积无血清培养基以抑制细胞增殖,从而确保实验结果主要反映细胞定向迁移。划痕后0,6,12 h,使用倒置相差显微镜观察并拍照,采用固定的相机参数记录图像,以保证采集结果的可比性和重复性。采用 Image J 软件分析划痕宽度,在同一区域选取多个测量点(n≥3),取平均划痕宽度,计算细胞迁移率。
细胞迁移率(%) = (初始划痕宽度–终末点划痕宽度) /初始划痕宽度× 100%
1.4.8 细胞实验相关检测方法
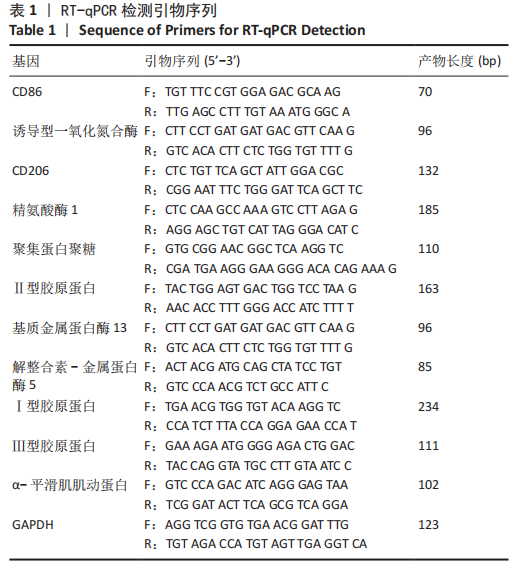
RT-qPCR检测:使用TRIzol试剂提取总RNA,利用NanoDrop ND-2000分光光度计测定RNA浓度和纯度。使用反转录试剂盒合成cDNA,将cDNA与SYBR Green PCR Master Mix、基因特异性引物及DEPC水混合,制备反应体系,在实时荧光定量PCR系统上进行扩增,以GAPDH作为内参基因。目标基因的相对表达量采用 2⁻ΔΔCt 方法进行计算。引物序列见表1。
免疫荧光染色:将细胞在25 ℃用40 g/L多聚甲醛固定
30 min,以保持细胞形态和结构完整;用0.1% TritonX-100处理5 min以增强细胞膜通透性,用封闭液孵育30min以减少非特异性结合;将一抗按1∶200稀释比加入封闭液中,在4 ℃下孵育过夜以充分结合靶蛋白;去除一抗后,PBS洗涤细胞3次,在25 ℃避光条件下与AlexaFluor488或594标记的二抗(1∶500)孵育1 h;用DAPI(1 µg/mL)染色细胞核5 min,在显微镜下采集荧光图像,随机选择至少3个视野进行观察和记录,采用 Image J软件分析荧光强度。
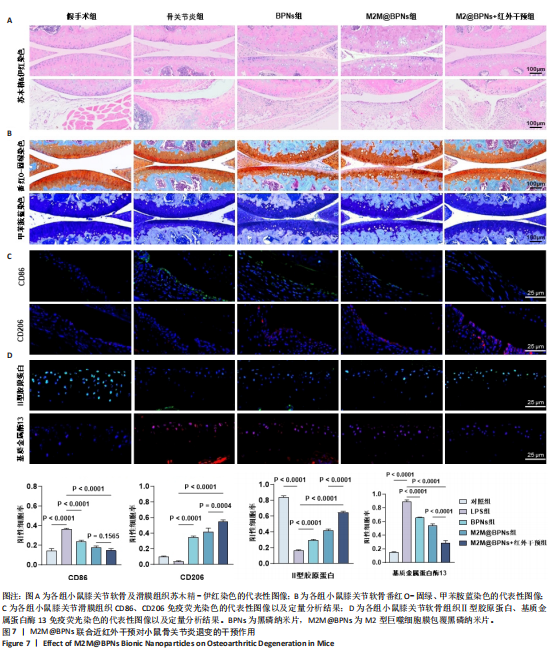
1.4.9 M2M@BPNs联合近红外干预对小鼠骨关节炎退变的干预作用 将30只C57BL/6小鼠随机分为假手术组、骨关节炎组、BPNs组、M2M@BPNs组、M2M@BPNs+近红外干预组,每组6只。切断骨关节炎组、BPNs组、M2M@BPNs组、M2M@BPNs+红外干预组小鼠右膝内侧半月板胫骨韧带建立骨关节炎模型[33],假手术组进行假手术(仅暴露关节而不切断韧带)。术后碘伏消毒切口,肌肉注射青霉素(5×104 IU/kg)抗感染。术后1周,骨关节炎组膝关节腔注射20 µL PBS,BPNs组和M2M@BPNs组膝关节腔分别注射20µL的BPNs分散液(100 µg/mL)、M2M@BPNs分散液(100 µg/mL),M2M@BPNs+近红外干预组膝关节腔注射20 µL M2M@BPNs分散液(100 µg/mL)24 h后接受近红外光照射(808 nm,0.75 W/cm²,180 s)3次,每次间隔30 s,每周治疗1次。术后8周,吸入异氟烷麻醉后颈椎脱臼处死小鼠,收集心脏、肝脏、脾脏、肺、肾及膝关节用于后续分析。
体内毒性分析:用冰冷PBS冲洗去除心脏、肝脏、脾脏、肺、肾组织残余血液,在4 ℃条件下用10%中性甲醛固定48 h;依次通过梯度浓度(体积分数70%,80%,95%,100%)乙醇脱水,二甲苯透明处理后包埋于石蜡中,使用旋转切片机切取4 µm连续切片,安装在聚-L-赖氨酸涂层载玻片上并于37 ℃干燥过夜;切片脱蜡复水后,先用苏木精染色5 min,再用伊红染色2 min,
经脱水并用合成树脂封固后,在光学显微镜下进行组织病理学观察与评估,包括炎症、坏死、纤维化及其他组织结构异常。
组织学染色:膝关节标本经梯度浓度乙醇脱水、二甲苯过夜透明、石蜡包埋后,使用切片机沿矢状面切成6 μm厚薄片放于载玻片上,经二甲苯脱石蜡、梯度浓度乙醇复水后,分别进行软骨与滑膜苏木精-伊红染色、软骨番红O-固绿和甲苯胺蓝染色液染色,中性树胶封固,光学显微镜下观察并拍摄图像。
免疫荧光染色:膝关节软骨与滑膜组织切片经二甲苯脱石蜡、梯度浓度乙醇复水后用体积分数3% H₂O₂阻断内源性过氧化物酶活性15 min,在37 ℃条件下用胰酶-EDTA进行抗原修复;经1.5%山羊血清封闭后,在4 ℃下与一抗(滑膜组织:CD206、CD86,软骨组织:Ⅱ型胶原蛋白,基质金属蛋白酶13,稀释比均为1∶200)孵育过夜;去除一抗,与AlexaFluor488/
594结合的二抗(1∶500)在25 ℃避光孵育1 h,用DAPI进行核染色,倒置显微镜下观察并拍摄图像,通过Image J软件分析阳性细胞率。
1.5 主要观察指标 M2M@BPNs联合红外干预在巨噬细胞极化重编程中的作用,M2M@BPNs联合红外干预对骨关节炎软骨细胞基质稳态的间接调控,M2M@BPNs联合红外干预对骨关节炎成纤维细胞代谢稳态的间接调控,M2M@BPNs联合红外干预对小鼠骨关节炎退变的干预作用。
1.6 统计学分析 采用 GraphPad Prism 9.5软件进行统计分析,定量数据均以均值±标准误表示,多组比较采用单因素方差分析(one-way ANOVA),两组比较采用 Student’s t 检验。以 P < 0.05 作为差异有显著性意义的判定标准。该文所统计学方法已获得苏州大学生物统计学专家的审核确认。