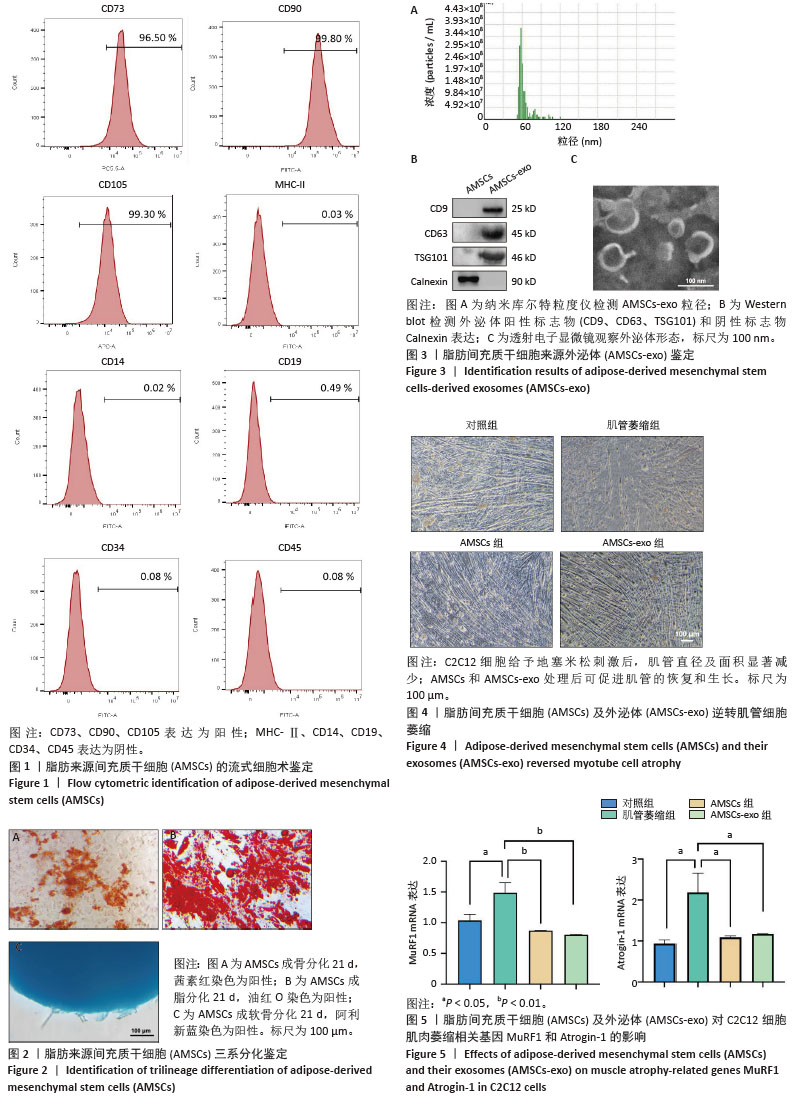
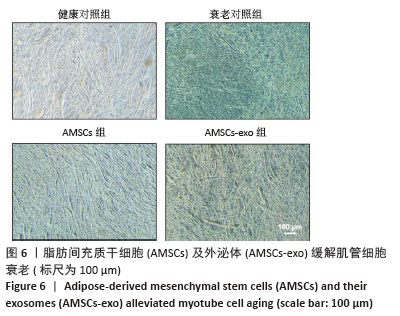
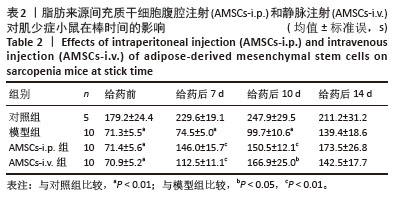
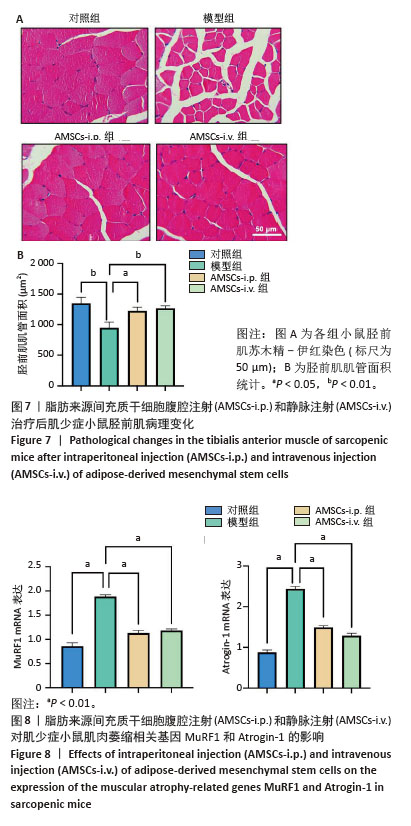
[1] CRUZ-JENTOFT AJ, BAEYENS JP, BAUER JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-423.
[2] Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr. 1989;50(5 Suppl): 1121-1235.
[3] CHEN LK, WOO J, ASSANTACHAI P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300-307.e2.
[4] 中华医学会骨质疏松和骨矿盐疾病分会. 肌少症共识[J].中华骨质疏松和骨矿盐疾病杂志,2016,9(3):215-227.
[5] 崔华,王朝晖,吴剑卿,等.老年人肌少症防控干预中国专家共识(2023)[J].中华老年医学杂志,2023,42(2):144-153.
[6] ANKER SD, MORLEY JE, VON HAEHLING S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512-514.
[7] MANKHONG S, KIM S, MOON S, et al. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells. 2020;9(6):1385.
[8] NAJM A, MOLDOVEANU ET, NICULESCU AG, et al. Advancements in Drug Delivery Systems for the Treatment of Sarcopenia: An Updated Overview. Int J Mol Sci. 2024;25(19):10766.
[9] FLACK KD, DAVY KP, HULVER MW, et al. Aging, resistance training, and diabetes prevention. J Aging Res. 2010;2011:127315.
[10] PIAO L, HUANG Z, INOUE A, et al. Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice. Stem Cell Res Ther. 2022; 13(1):226.
[11] SONG J, LIU J, CUI C, et al. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J Cachexia Sarcopenia Muscle. 2023;14(2):915-929.
[12] MAHINDRAN E, LAW JX, NG MH, et al. Mesenchymal Stem Cell Transplantation for the Treatment of Age-Related Musculoskeletal Frailty. Int J Mol Sci. 2021;22(19):10542.
[13] KUROSAWA T, IKEMOTO-UEZUMI M, YOSHIMOTO Y, et al. Tissue-specific functions of MSCs are linked to homeostatic muscle maintenance and alter with aging. Aging Cell. 2024;23(11):e14299.
[14] WOSCZYNA MN, KONISHI CT, PEREZ CARBAJAL EE, et al. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019;27(7):2029-2035.e5.
[15] ZHU Y, HUANG C, ZHENG L, et al. Safety and efficacy of umbilical cord tissue-derived mesenchymal stem cells in the treatment of patients with aging frailty: a phase I/II randomized, double-blind, placebo-controlled study. Stem Cell Res Ther. 2024;15(1):122.
[16] YOUSEFI K, RAMDAS KN, RUIZ JG, et al. The Design and Rationale of a Phase 2b, Randomized, Double-Blinded, and Placebo-Controlled Trial to Evaluate the Safety and Efficacy of Lomecel-B in Older Adults with Frailty. J Frailty Aging. 2022;11(2):214-223.
[17] NGUYEN NT, PHAN HT, LE PM, et al. Safety and efficacy of autologous adipose tissue-derived stem cell transplantation in aging-related low-grade inflammation patients: a single-group, open-label, phase I clinical trial. Trials. 2024;25(1):309.
[18] GOLPANIAN S, DIFEDE DL, KHAN A, et al. Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty. J Gerontol A Biol Sci Med Sci. 2017;72(11):1505-1512.
[19] TOMPKINS BA, DIFEDE DL, KHAN A, et al. Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci. 2017;72(11):1513-1522.
[20] SHEHATA AS, AL-GHONEMY NM, AHMED SM, et al. Effect of mesenchymal stem cells on induced skeletal muscle chemodenervation atrophy in adult male albino rats. Int J Biochem Cell Biol. 2017;85:
135-148.
[21] LI TS, SHI H, WANG L, et al. Effect of Bone Marrow Mesenchymal Stem Cells on Satellite Cell Proliferation and Apoptosis in Immobilization-Induced Muscle Atrophy in Rats. Med Sci Monit. 2016;22:4651-4660.
[22] TAKEGAKI J, SASE K, KONO Y, et al. Intramuscular injection of mesenchymal stem cells augments basal muscle protein synthesis after bouts of resistance exercise in male mice. Physiol Rep. 2024;12(7): e15991.
[23] ARCHACKA K, GRABOWSKA I, MIERZEJEWSKI B, et al. Hypoxia preconditioned bone marrow-derived mesenchymal stromal/stem cells enhance myoblast fusion and skeletal muscle regeneration. Stem Cell Res Ther. 2021;12(1):448.
[24] WANG QQ, JING XM, BI YZ, et al. Human Umbilical Cord Wharton’s Jelly Derived Mesenchymal Stromal Cells May Attenuate Sarcopenia in Aged Mice Induced by Hindlimb Suspension. Med Sci Monit. 2018;24: 9272-9281.
[25] WANG C, ZHAO B, ZHAI J, et al. Clinical-grade human umbilical cord-derived mesenchymal stem cells improved skeletal muscle dysfunction in age-associated sarcopenia mice. Cell Death Dis. 2023;14(5):321.
[26] 陈三,杨润泽,吴家媛.预处理来源外泌体在细胞增殖分化及凋亡中的作用[J].中国组织工程研究,2023,27(19):3029-3039.
[27] MAHINDRAN E, WAN KAMARUL ZAMAN WS, AHMAD AMIN NOORDIN KB, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Hype or Hope for Skeletal Muscle Anti-Frailty. Int J Mol Sci. 2023;24(9):7833.
[28] LO SICCO C, REVERBERI D, BALBI C, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl Med. 2017;6(3):1018-1028.
[29] FIGLIOLINI F, RANGHINO A, GRANGE C, et al. Extracellular Vesicles From Adipose Stem Cells Prevent Muscle Damage and Inflammation in a Mouse Model of Hind Limb Ischemia: Role of Neuregulin-1. Arterioscler Thromb Vasc Biol. 2020;40(1):239-254.
[30] WANG C, SONG W, CHEN B, et al. Exosomes Isolated From Adipose-Derived Stem Cells: A New Cell-Free Approach to Prevent the Muscle Degeneration Associated With Torn Rotator Cuffs. Am J Sports Med. 2019;47(13):3247-3255.
[31] NAKAMURA Y, MIYAKI S, ISHITOBI H, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11): 1257-1265.
[32] LI Z, LIU C, LI S, et al. BMSC-Derived Exosomes Inhibit Dexamethasone-Induced Muscle Atrophy via the miR-486-5p/FoxO1 Axis. Front Endocrinol (Lausanne). 2021;12:681267.
[33] SANZ-ROS J, ROMERO-GARCÍA N, MAS-BARGUES C, et al. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci Adv. 2022;8(42):eabq2226.
[34] DAI H, ZHENG W, LUO J, et al. Inhibiting uptake of extracellular vesicles derived from senescent bone marrow mesenchymal stem cells by muscle satellite cells attenuates sarcopenia. J Orthop Translat. 2022;35:23-36.
[35] KARTIKA RW, SIDHARTA VM, DJUARTINA T, et al. New Insight in Using of Mesenchyme Stem Cell Conditioning Medium for the Impaired Muscle related Biomarkers: In vivo Study with Rat Model. Ann Afr Med. 2024;23(4):674-679.
[36] WANG L, JIAO XF, WU C, et al. Trimetazidine attenuates dexamethasone-induced muscle atrophy via inhibiting NLRP3/GSDMD pathway-mediated pyroptosis. Cell Death Discov. 2021;7(1):251.
[37] CRUZ-JENTOFT AJ, LANDI F, TOPINKOVÁ E, et al. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13(1):1-7.
[38] GE J, ZENG J, MA H, et al. A New Index Based on Serum Creatinine and Cystatin C Can Predict the Risks of Sarcopenia, Falls and Fractures in Old Patients with Low Bone Mineral Density. Nutrients. 2022;14(23):5020.
[39] BURGEL CF, CARVALHO BZO, MILESI BM, et al. SARC-CalF using calf circumference adjusted for BMI predicts 6-mo readmission and mortality in hospitalized patients: a secondary analysis of a cohort study. Am J Clin Nutr. 2025;121(1):151-157.
[40] HEN LK, LEE WJ, PENG LN, et al. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2016;17(8):767.e1-7.
[41] BOWEN TS, SCHULER G, ADAMS V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2015;6(3):197-207.
[42] ZHIDU S, YING T, RUI J, et al. Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. Stem Cell Res Ther. 2024;15(1):266.
[43] HOANG DM, PHAM PT, BACH TQ, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1):272.
[44] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
[45] WIDJAJA G, JALIL AT, BUDI HS, et al. Mesenchymal stromal/stem cells and their exosomes application in the treatment of intervertebral disc disease: A promising frontier. Int Immunopharmacol. 2022; 105:108537.
[46] CUDKOWICZ ME, LINDBORG SR, GOYAL NA, et al. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve. 2022;65(3):291-302.
[47] WELSH JA, GOBERDHAN DCI, O’DRISCOLL L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404.
[48] MA H, JING Y, ZENG J, et al. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate muscle atrophy via the miR-132-3p/FoxO3 axis. J Orthop Translat. 2024;49:23-36.
[49] WIAFE B, KADAM R, METCALFE PD. Intraperitoneal administration of mesenchymal stem cells is effective at mitigating detrusor deterioration after pBOO. Am J Physiol Renal Physiol. 2020;318(3): F549-F556.
[50] BODINE SC, BAEHR LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307(6):E469-484. |