中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (4): 936-948.doi: 10.12307/2025.963
• 组织构建综述 tissue construction review • 上一篇 下一篇
组织工程治疗策略在口腔黏膜下纤维化中的研究与应用进展
余诗宇,俞苏桐,徐 杨,镇祥燕,韩凤选
- 苏州大学骨科研究所,江苏省苏州市 215006
-
收稿日期:2024-11-12接受日期:2024-12-20出版日期:2026-02-08发布日期:2025-05-20 -
通讯作者:韩凤选,博士,教授,苏州大学骨科研究所,江苏省苏州市 215006 -
作者简介:余诗宇,女,2002年生,重庆市人,汉族,苏州大学本科在读,主要从事口腔生物医用材料、药物控释材料、口腔黏膜病、口腔组织工程研究。 -
基金资助:江苏省杰出青年基金项目(BK20240020),项目负责人:韩凤选
Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis
Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan
- Orthopedics Institution of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2024-11-12Accepted:2024-12-20Online:2026-02-08Published:2025-05-20 -
Contact:Han Fengxuan, MD, Professor, Orthopedics Institution of Soochow University, Suzhou 215006, Jiangsu Province, China -
About author:Yu Shiyu, Orthopedics Institution of Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:Jiangsu Outstanding Youth Fund, No. BK20240020 (to HFX)
摘要:
文题释义:
口腔黏膜下纤维化:是一种具有较高恶性转化潜力的慢性疾病,可侵犯口腔的任何部位。由于固有层的纤维组织变性和上皮萎缩,从而引起黏膜硬化,形成条索,最终导致牙关紧闭,妨碍口腔各种功能的发挥。
组织工程:是一门以细胞生物学和材料科学相结合进行体外或体内构建组织或器官的新兴学科。
背景:口腔黏膜下纤维化是一种容易恶变的慢性进行性疾病,传统治疗手段效果不理想,且存在局限性。组织工程作为一门新兴学科,为口腔黏膜下纤维化的治疗开辟了新路径。
目的:综述口腔黏膜下纤维化发病机制以及治疗的最新进展,总结并分析间充质干细胞、生物支架材料、组织工程口腔黏膜在口腔黏膜下纤维化中的作用与研究进展,为组织工程方法治疗口腔黏膜下纤维化的研究及临床应用提供思路。
方法:第一作者于2024年10月应用计算机在PubMed和中国知网数据库检索1970年1月至2024年10月相关文献,以“oral submucous fibrosis,tissue engineering,mesenchymal stem cells,bioscaffold materials”为英文检索词,以“口腔黏膜下纤维化,组织工程,间充质干细胞,生物支架材料”为中文检索词,最终纳入166篇文献进行分析。
结果与结论:①口腔黏膜下纤维化的发病机制复杂,许多因素与口腔黏膜下纤维化密切相关,但最终都通过促进胶原蛋白沉积并加速成纤维细胞增殖来促进口腔黏膜下纤维化的发展;②传统口腔黏膜下纤维化治疗手段存在患者依从性低、效果不理想等问题,急需新的治疗策略;③间充质干细胞因免疫调节、抗氧化特性,调控病理微环境,减轻炎症并抑制纤维化进程,为口腔黏膜下纤维化治疗提供新思路;④生物材料作为药物和细胞递送载体,调节病理微环境,在各类纤维化疾病中得以应用,为口腔黏膜下纤维化的治疗提供新方案;⑤组织工程口腔黏膜可作为自体黏膜替代物来促进组织修复,也为疾病模型的建立提供了基础;⑥组织工程治疗策略对于口腔黏膜下纤维化的综合治疗潜力巨大,现已有一些研究将间充质干细胞与生物材料用于治疗口腔黏膜下纤维化,但仍需进一步完善与创新,对于未来探索基于组织工程化的口腔黏膜下纤维化治疗方案具有重要意义。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
余诗宇, 俞苏桐, 徐 杨, 镇祥燕, 韩凤选. 组织工程治疗策略在口腔黏膜下纤维化中的研究与应用进展[J]. 中国组织工程研究, 2026, 30(4): 936-948.
Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948.
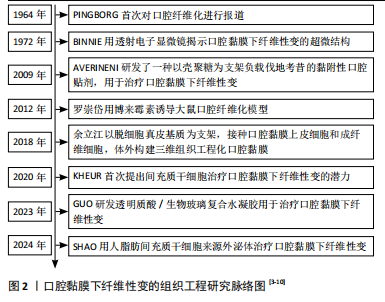
2.2 OSF的发病因素 OSF的发病机制较为复杂,目前研究表明,多种因素均与OSF的发生密切相关。这些因素最终都通过促进胶原蛋白的形成和沉积以及加速成纤维细胞的增殖来促进OSF的发生与发展[1]。OSF的主要致病因素,见图3[11]。
2.2.1 槟榔 大量研究已经证实,咀嚼槟榔与OSF的发生密切相关[1,12-14]。槟榔是热带棕榈科植物槟榔树的果实,因具有轻微的兴奋作用、欣快感、提升注意力以及促进消化等效果,在部分人群中广受欢迎。槟榔中所含的多种生物碱是诱发OSF的重要因素。槟榔碱是槟榔的主要生物碱成分[15],具有遗传毒性和致突变性,能够影响基因表达。槟榔碱通过上调参与胶原蛋白修饰的热休克蛋白47[16]、丝聚蛋白和分泌型卷曲相关蛋白1等基因的表达[17],加速OSF的发生发展。此外,槟榔碱可通过多种途径上调细胞内活性氧水平,诱导口腔黏膜上皮细胞和成纤维细胞产生过氧化氢和超氧自由基,进而激活NADPH氧化酶(NOX)1和4,加剧DNA损伤[18-20]。在一氧化氮存在的情况下,槟榔中的生物碱能够促进N-亚硝胺代谢物的生成,进一步增加细胞毒性和遗传毒性[21-22]。
此外,槟榔中含有的铜元素能够激活多种血管生成因子[23-24],促进内皮细胞的激活与增殖,可能在OSF的发生中发挥重要作用[25]。槟榔产品中含有的氟化物也可能是引发OSF的潜在因素之一[26]。
2.2.2 免疫异常 OSF作为一种具有癌变倾向的口腔黏膜疾病,伴随着显著的免疫微环境变化,包括免疫细胞的浸润以及免疫相关细胞因子的表达上调。研究显示,在OSF患者的病变组织中有大量炎症细胞的浸润[27]。近期研究表明,T细胞亚群的比例失衡可能会导致自体免疫反应的增强[28-30],从而加速纤维化进程。此外,巨噬细胞和淋巴细胞等免疫细胞会释放肿瘤坏死因子α、白细胞介素6、转化生长因子β等多种细胞因子和生长因子[31],能够刺激成纤维细胞增殖和胶原合成,进而引发组织的纤维化反应。此外,免疫细胞分泌的趋化因子能促进成纤维细胞的迁移和增殖[32],增强细胞外基质的合成[33]。因此,针对免疫反应的调节可能成为OSF治疗的新策略。
2.2.3 表观遗传调控与遗传易感性 表观遗传调控机制在OSF中也起关键作用。Wnt抑制因子1在OSF恶化过程中通过启动子甲基化而被下调或沉默,成为口腔鳞状细胞癌早期检测的潜在表观遗传生物标志物[34]。此外,OSF患者的颊细胞和唾液中p16基因的启动子甲基化水平显著高于健康对照组,显示了p16高甲基化与OSF的关联性[35]。通过Infinium HumanMethylation450 BeadChip Array分析7例OSF组织样本和5例对照样本的DNA甲基化变化,结合转录组数据,研究者鉴定出38个高甲基化下调基因和55个低甲基化上调基因,并通过甲基化特异性和定量RT-PCR验证了FGF13、RPS6KA3和ACSL4基因的异常低甲基化及高表达[36],进一步支持表观遗传调控在OSF中的关键作用。
除了表观遗传因素外,特定基因突变与异常表达也可能与OSF的发生密切相关。研究者发现OSF患者中HLA-DQB10502等位基因的频率显著高于健康对照组,且该等位基因与OSF的发生呈正相关[37],这一结果表明HLA-DQB10502可能是OSF的易感基因。此外,MANNAN等[38]通过分析健康人群与OSF患者的GSTM1和GSTT1基因型,发现OSF患者GSTM1和GSTT1缺失基因型的频率高于对照组,且GSTT1基因缺失型与OSF显著相关,这表明携带这种基因型的个体可能更容易发展为OSF,进一步说明遗传因素可能在OSF的发病机制中发挥重要作用。另外,有研究通过挖掘综合数据库,识别出945个差异表达基因[39],这些基因在OSF患者与正常对照组之间表现出显著的差异。这些研究结果均支持了遗传易感性在OSF发病中的关键作用。
2.3 口腔黏膜下纤维化的病理生理学改变
2.3.1 细胞因子及生长因子水平的改变 口腔黏膜损伤会引发上皮细胞的炎症反应,进而激活巨噬细胞和T细胞分泌细胞因子。多项研究表明,OSF患者血清中的细胞因子水平与正常人相比发生显著变化,例如,白细胞介素1、白细胞介素6和转化生长因子β1等促纤维化细胞因子水平明显升高[40-43],其中转化生长因子β发挥着关键作用[44-47]。此外,肿瘤坏死因子α水平也有所升高[48],这一因子可促进颊黏膜成纤维细胞增殖和胶原合成。这些机制共同导致局部胶原的过度沉积和成纤维细胞的增殖,从而推动纤维化的进展,见图4[11]。
2.3.2 氧化应激及血管的改变 刺激因素引起的缺氧是局部组织纤维化形成的主要机制之一。局部组织的氧供应不足或氧负荷增加都会导致缺氧,而体内的氧浓度与低氧诱导因子1α的表达密切相关[49-50]。在缺氧状态下,低氧诱导因子1α的表达上调,进一步通过增加纤溶酶原激活物抑制剂的表达,促进口腔黏膜下层细胞外基质的沉积,从而引发纤维化[51-52]。此外,研究显示OSF患者低氧诱导因子1α的高表达与恶性转化的高风险密切相关[53]。
研究表明,在纤维化进展中,组织内血管数量逐渐减少可能与血栓素1水平上调有关[54],从而造成组织缺氧。然而,也有研究发现,OSF组织中血管管腔的平均密度和直径高于正常黏膜[55],在OSF患者中,低氧诱导因子1α[56-57]、血管内皮生长因子等细胞因子表达水平升高可能是OSF局部缺氧环境引起的一种代偿性反应[58-60]。血管
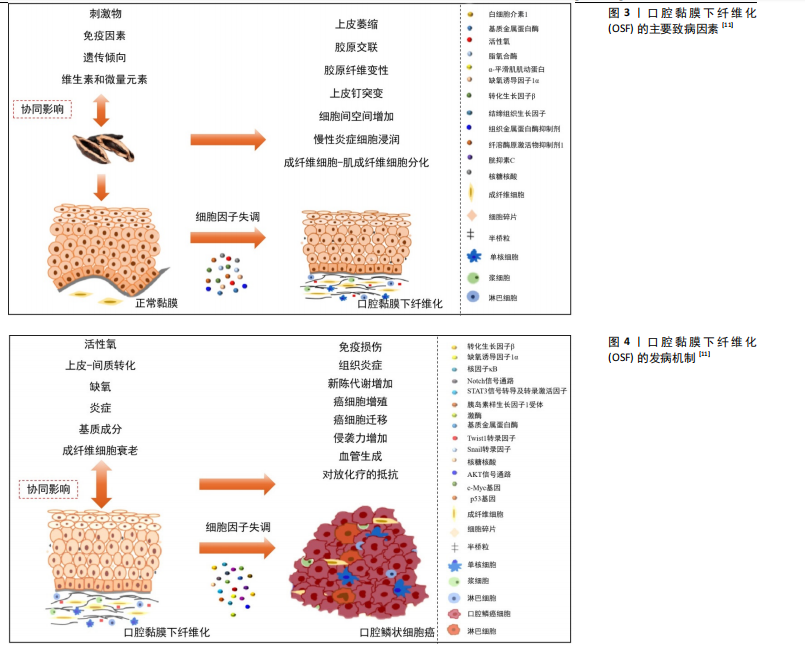
数量的增加或减少也可能与OSF向口腔鳞状细胞癌发展的进程相关,这一现象也有助于区分OSF的不同阶段及其向口腔鳞状细胞癌发展的潜在风险。
2.3.3 上皮-间质转化 上皮-间质转化是指上皮细胞从紧密相连且非运动状态转化为连接疏松并具有迁移活性的间质组织的过程。大量文献表明,上皮-间质转化在正常细胞恶变成为癌细胞,以及癌细胞转移到其他部位时均起着重要作用[61-63]。近年来,研究也发现上皮-间质转化同样也发生在癌前病变OSF中。在OSF组织中,上皮-间质转化生物标志物显著变化,例如波形蛋白表达显著升高,而钙黏蛋白E表达显著降低[64-66]。XIE等[67]通过体外实验进一步验证了这一过程,即槟榔碱可上调PA28γ和磷酸化MEK1蛋白水平,并促进上皮细胞发生上皮-间质转化。此外,基质硬度增加显著促进了上皮细胞的上皮-间质转化。将硬质胶原基质植入大鼠口腔黏膜后,发现上皮细胞中上皮-间质转化相关标志物显著变化,并伴随Piezo1和YAP表达增加[64],在OSF发展中发挥了重要作用。
2.4 OSF的传统治疗方式及不足 OSF给患者带来了身心的双重折磨,尤其严重影响了患者的咀嚼和开口功能。由于目前尚无明确的根治方法,因此在早期对轻度OSF患者进行干预,减轻痛苦并预防癌变显得尤为重要。近年来,研究者积极探索OSF的预防和治疗方法,现有的治疗手段主要包括保守治疗和手术治疗。保守治疗又可分为药物疗法和物理疗法。
2.4.1 药物疗法 目前,药物疗法是OSF的主要治疗手段,基本原理在于通过调节炎症因子与促细胞外基质降解[68-69],从而达到抗纤维化的效果。糖皮质激素[70-71]、干扰素γ[72]、秋水仙碱等药物通过控制炎症因子、改善炎症反应、减少胶原过度形成[73],从而提高张口度、缓解灼烧感。蛋白水解酶类药物(如透明质酸酶、糜蛋白酶)可通过降解透明质酸和胶原蛋白等细胞外基质,降低黏度,进而改善患者的开口度[74-76]。
改善局部缺氧也是OSF药物治疗的重要机制之一。研究表明,番茄红素[77-79]、螺旋藻等具有良好的抗氧化和清除自由基的作用[80-82]。多项临床试验已证实,这些抗氧化剂在缓解张口困难、减轻灼烧感以及改善脸颊柔韧性方面具有显著疗效。研究表明,中药在改善开口度方面疗效显著[83]。一些天然药物,如姜黄素[84-87]、丹参[88-90]、芦荟[91-93],不仅可以起抗炎作用,还可以改善缺氧。
然而,现有的药物应用方式仍较为传统,如注射药物依从性较低,口服药物可能引发全身反应,局部涂抹药物则易受到唾液的影响。如何将药物与新型治疗手段结合,以提升疗效,是未来研究的重要方向。
2.4.2 物理疗法
(1)高压氧疗法:缺血、缺氧是OSF发病的机制之一,高压氧疗法通过利用氧浓度与扩散梯度之间的直接关系,将高于局部大气压的纯氧输送到更深的组织,从而改善组织缺氧,促进血管新生,这种方法有助于增加组织氧、促进新陈代谢,从而减少氧化应激并加速伤口愈合,明显恢复软组织的柔软性,并改善患者的张口程度[94]。此外,高压氧疗法还能够调控细胞因子和生长因子水平,从而抑制成纤维细胞的活化和上皮-间充质转化[95]。高压氧疗法还可以减少促炎因子的产生[96-97],如肿瘤坏死因子α、白细胞介素1和白细胞介素6,同时增加抗炎因子白细胞介素10的产生,从而改善OSF局部的免疫微环境,有利于OSF的治疗。但在临床应用中,高压氧疗法需要长期重复治疗,并且对重度OSF的疗效不理想[4]。
(2)张口训练:研究表明,张口训练在OSF患者术后能够有效改善张口受限[98],并且将张口训练与药物治疗结合使用,能够显著增加患者的张口度[99]。张口训练作为其他治疗方法的补充手段可增强整体治疗效果。
2.4.3 手术治疗 手术治疗主要适用于局部明显纤维化形成条索、开口度小于20 mm的重度OSF患者。通过外科手术的方法,切除纤维条索病变,随后选择血管化良好的皮瓣进行移植并联合药物治疗[100]。然而,由于手术治疗具有较大的侵入性并且会造成二次创伤,这一方法并不是大多数OSF患者的第一选择。
2.5 组织工程治疗口腔纤维化的前景及应用 组织工程以开发具有正常生理结构与功能类似的各种病损组织的替代物为目标[101],基本原理是将种子细胞、生物材料、生长因子与适宜的外部物理环境合理组合,在体外模拟体内的组织微环境,构建组织样结构,再将其植入病损部位实现修复与再生[102]。严重创伤、先天畸形和大型肿瘤切除手术导致口腔黏膜缺损,无法进行一期缝合。而受损的口腔环境中存在微生物感染、体液大量流失、异物污染、伤口挛缩和二期愈合时瘢痕形成的风险[103]。因此,使用组织或生物材料移植物填补这些大块缺损,以防止严重的功能缺陷,如咀嚼,吞咽和言语困难,并且促进适当的伤口愈合至关重要。近年来,组织工程治疗策略在其他各类纤维化疾病中的研究成为热点,给OSF的研究带来启发与灵感,为OSF的综合治疗开辟了新路径,有望弥补OSF传统治疗方式的不足,为OSF的治疗提供新的思路,见图5。
2.5.1 种子细胞 近年来,细胞移植疗法在各类纤维化疾病中的应用研究是一大热点。常用于纤维化疾病研究的细胞种类包括全能干细胞中的胚胎干细胞、脂肪间充质干细胞、骨髓间充质干细胞等多能干细胞,以及成肌细胞等单能干细胞。研究证实,来源于胚胎干细胞的免疫和基质
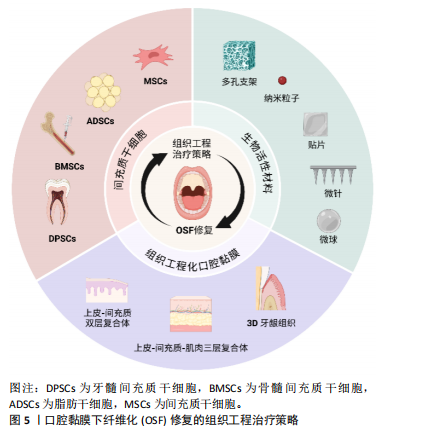
调节细胞能通过静脉输送到达肺部,抑制肺损伤小鼠的肺部炎症和纤维化[104]。一些祖细胞和成体细胞也可用于纤维化疾病的治疗。例如,由肝祖细胞产生的自噬调节外泌体miRNA 可抑制血吸虫病的肝纤维化[105]。此外,成肌细胞在OSF的治疗中也有研究。有学者从人的骨骼肌中提取得到成肌细胞,将其移植到OSF小鼠病损颊黏膜处,成肌细胞通过介导旁分泌效应增强细胞外基质重塑并促进组织再生,在体内证实了对OSF的抑制作用[106]。另一方面,成纤维细胞、角质形成细胞等可作为种子细胞[107],联合不同种类的支架材料用于构建组织工程化口腔黏膜。然而,间充质干细胞是目前在治疗纤维化疾病中应用较广且潜力较大的细胞种类。在OSF治疗策略中,对于间充质干细胞的开发与应用研究具有重要意义。
OSF主要是由慢性炎症反应、氧化应激、缺氧、微血管减少以及转化生长因子β1通路介导的肌成纤维细胞过度增殖等因素直接作用的结果。间充质干细胞是一类具有多向分化潜能、自我更新能力的成体干细胞,因不存在重大伦理问题、低免疫原性和具有免疫调节能力等特点[108-109],在肝纤维化[110]、肠道纤维化[111]、肺纤维化[112-113]、角膜瘢痕等各类纤维化疾病研究中已成为重要的候选细胞[114-115]。
KHEUR等[7]从间充质干细胞的免疫调节特性、抗纤维化、抗氧化、血管生成潜力等方面讨论了间充质干细胞用于治疗OSF的可行性。
(1)免疫调节特性:当免疫系统失活时,间充质干细胞可以促进炎症反应,当免疫系统过度激活时可以抑制炎症反应,以避免自我过度攻击[108]。间充质干细胞可通过分泌白细胞介素10等细胞因子或直接作用于T细胞抑制干扰素γ和肿瘤坏死因子α的分泌,上调白细胞介素4,调控免疫微环境[116],减轻由炎症反应引起的转化生长因子β1通路激活。最新的研究表明,局部注射牙髓间充质干细胞能够调节OSF病损中KRT19+MIF+上皮细胞数量与上皮-T细胞的相互作用,从而减少胶原沉积和促进血管再生,达到优于糖皮质激素治疗的效果[28]。有研究发现,利用间充质干细胞的归巢特性,与间充质干细胞和Ⅰ型胶原酶修饰的载有尼达尼布的脂质体之间的生物共轭作用(MSCs-Lip@NCAF)构建了一个基于间充质干细胞的纳米工程平台,用于治疗严重纤维化有很大的潜力[117]。此外,骨髓间充质干细胞可通过旁分泌途径下调转化生长因子β1、白细胞介素6等促纤维化因子抑制细胞外基质的分泌[118-120],并且骨髓间充质干细胞培养液上清在转录和翻译水平上显著降低人瘢痕成纤维细胞中结缔组织生长因子、纤溶酶原激活因子抑制剂1、转化生长因子β1等促纤维化基因的表达,增强转化生长因子β3和核心蛋白聚糖等抗纤维化基因的表达[119]。脐带间充质干细胞可通过产生外泌体阻断转化生长因子β1/Smad信号通路,抑制肝细胞上皮-间充质转化[121-122]。脂肪来源干细胞分泌的多种细胞因子能降低Ⅰ型、Ⅲ型胶原和α-平滑肌肌动蛋白的表达[123],实现细胞外基质的重塑。因此,间充质干细胞的免疫调节特性能够针对OSF异常的免疫微环境发挥作用,可下调局部炎症因子并分泌促炎因子,抑制细胞外基质的沉积。
(2)抗氧化特性:间充质干细胞的抗氧化作用能够通过多种途径来实现。间充质干细胞能够分泌大量血管内皮生长因子、成纤维细胞生长因子2等血管生成因子促进血管生成,并且上调超氧化物歧化酶、谷胱甘肽过氧化物酶等氧自由基清除相关酶的表达,清除活性氧,对抗缺氧和氧化应激[124-125]。间充质干细胞来源的富含miR-100a-5p的外泌体,通过递送miR-100-5p来抑制靶基因NADPH氧化酶4的表达水平,从而减少活性氧的产生并通过Nox4-ROS-Nrf2轴减轻氧化应激[126]。还有研究表明间充质干细胞通过HIF1A-HMOX1轴介导的自噬激活抑制活性氧的产生[127]。已有研究证实,牙龈来源间充质干细胞通过抑制白细胞介素1β、白细胞介素8、肿瘤坏死因子α等炎症因子基因的表达水平[128],上调谷胱甘肽过氧化物酶与超氧化物歧化酶来减轻博来霉素诱导的肺纤维化、肺部炎症、肺水肿和细胞凋亡。
因此,间充质干细胞作为治疗各种纤维疾病的重要候选细胞,不仅能迁移到病损部位分化为相应的组织细胞,促进组织再生,还可多方面综合调控病损部位的组织微环境。因此,间充质干细胞在抑制OSF发生,甚至逆转OSF病程的治疗中具有很好的应用前景。
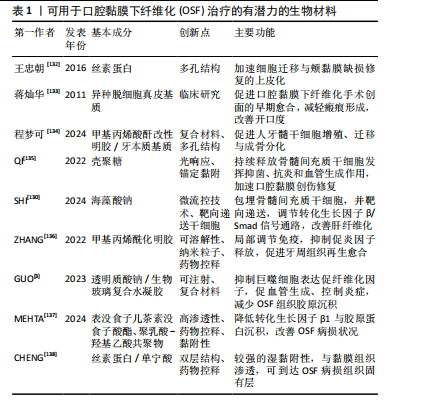
2.5.2 生物支架材料 生物支架材料能为细胞存活、增殖、分化、迁移提供适宜的微环境,在组织再生修复中起着关键作用[129]。支架材料主要分为明胶、丝素蛋白、壳聚糖等天然生物材料与聚乳酸等高分子人工合成生物材料两大类,通过光交联、微流控、静电纺丝等技术组成多孔、微球[130]、微针、水凝胶等类型[131]。生物支架材料具有良好的生物相容性与可降解性,在组织工程中应用广泛。见表1[3,130,132-138]。
(1)作为细胞支架与载体:作为细胞支架与递送载体,
生物活性材料在组织工程中起着不可替代的作用。生物活性材料不仅可作为细胞载体局部投放种子细胞,并且有效将种子细胞控制在受损部位,高效发挥作用[139],还为细胞提供基质金属蛋白酶靶序列和RGD序列等黏附位点[140],为细胞的增殖、分化、迁移提供临时的三维支架,随后逐渐降解,被机体自身细胞外基质取代。相关研究表明,丝素蛋白支架能诱导上皮细胞迁移并按规律生长,促进大鼠口腔黏膜缺损愈合,脱细胞真皮基质能促进OSF的术后愈合,对减少瘢痕具有显著作用[132-133]。
近年来,生物活性材料在提高干细胞移植治疗效果方面取得了较大的进展,LI等[141-142]研制了一种封装了铜纳米酶和金纳米颗粒的活性氧响应性葡聚糖复合材料,不仅能够过释放铜按需清除活性氧,还可标记间充质干细胞作为CT成像示踪剂,提高了间充质干细胞的移植存活率,用于治疗肺纤维化。材料还能对间充质干细胞进行基因工程改造,同时调控和监测间充质干细胞的生物学行为[143]。
随着生物支架材料的发展,学者们不再局限于单一的支架材料,许多研究通过改变支架材料的形态、对特殊位点改性等方法提升了支架材料的功能。例如,改性水凝胶联合牙本质基质能构建具有特定孔隙率、力学强度及生物学性能的生物活性支架,有利于牙髓-牙本质再生[134]。Arg-Gly-Asp(RGD)修饰的羟基丁基壳聚糖水凝胶,能够增强骨髓间充质干细胞在水凝胶内的黏附和增殖,可以抑制瘢痕疙瘩成纤维细胞的增殖并减少瘢痕疙瘩组织的结节性胶原纤维[144]。胰岛素样生长因子1的C结构域肽修饰的壳聚糖活性水凝胶(CS-IGF-1C)增强了干细胞治疗急性心肌梗死的效果[145]。通过氨基和苯甲醛基团之间的可逆席夫碱连接制备了自修复能力的水凝胶,不仅能提高间充质干细胞的移植存活率,还可以通过调节胶原蛋白的合成来抑制纤维化[146]。QI等[135]受到口腔特有的创伤环境的启发,制备了一种缓释骨髓间充质干细胞的光响应抗菌水凝胶,其在避光条件下能成胶密封伤口并形成屏障,从而发挥抑菌、抗炎和血管生成作用;其在光照条件下能脱落并自行降解,从而快速修复口腔黏膜创伤。这些应用都能够为间充质干细胞用于治疗OSF提供新的启发。
(2)辅助调控病理微环境:生物活性材料作为很好的缓释系统,能够负载药物及生长因子等,实现高效递送与控释,达到局部治疗的作用。微针形式的水凝胶通过向牙周组织递送负载四环素与细胞因子的纳米粒子,诱导巨噬细胞极化并且抑制局部促炎因子,从而调控局部免疫微环境,促进组织愈合[136]。此外,使用微流控技术制备可注射的改性明胶微球作为载药系统,可实现药物的控释,对细胞外基质合成与降解进行精细调控。生物材料不仅能够作为单一的载药缓释系统,还能对细胞膜进行修饰,改善氧化应激微环境,增强间充质干细胞移植的效果[141]。WU等[147]研制的一种湿黏性水凝胶心脏贴剂能够通过改性后锚定在心脏表面,并且形成非黏性的顶面避免粘连周围组织,延长了药物和细胞的体内保留时间,从而促进血管生成,减少心肌纤维化。这样的锚定作用与非黏性顶面形成的单向干细胞递送系统在活动度较大且受唾液与牙齿活动影响的口腔环境中应用十分合适,或许能为OSF治疗提供有利帮助。
目前,已经有一些研究将生物材料应用于治疗OSF,例如GUO等[3]制备的可注射透明质酸钠-生物玻璃复合水凝胶可能通过硅酸盐离子抑制巨噬细胞表达转化生长因子β1、肿瘤坏死因子α等促纤维化因子,该材料的促血管生成活性用以对抗缺氧以及控制炎症反应,显著减少了大鼠OSF组织胶原沉积,但需多次长期注射,十分考验患者依从性。MEHTA等[137]通过计算机工具筛选出合适的药物,制备了一种黏附性的水凝胶贴剂,靶向作用于OSF病变位点,有效改善了OSF模型大鼠的张口度。CHENG等[138]制备的黏性丝素蛋白-单宁酸双层载药微针具有良好的生物相容性及药物控释性能,能够达到定向穿透和无痛给药目的,但缺乏体内效果验证。
(3)作为黏性敷料:水凝胶、静电纺丝纤维、薄膜、海绵等生物材料因生物相容性好、力学强度适中以及可降解、抗菌等性能,有益于减少瘢痕形成,逐步取代了纱布、棉绒等传统敷料[148]。向水凝胶骨架中加载抗生素[149]、没食子酸等能够赋予抗菌性能及活性氧清除功能[150],减少创面的炎症反应,加速皮肤组织重建,促进愈合。ZHAO等[151-152]添加纳米银等金属材料,相对于传统抗菌药物,抗菌谱广且不易产生耐药性。此外,可注射类的黏性辅料可填补不规则伤口[150],不仅能作为保护性屏障,还具有高保水性以及高透氧性,从而促进伤口修复[149]。这为改善OSF手术治疗的预后具有十分重要的意义。
与皮肤烧伤伤口要求敷料易于剥离减少炎症粘连导致的二次损伤不同,用于口腔黏膜的伤口敷料需要克服口腔内的机械活动、唾液冲洗、温度变化等复杂特殊的物理化学环境。因此,研究与探索具有黏附性、韧性、与自愈合能力的敷料一直以来都是治疗口腔黏膜疾病的一大热点与难点[153]。ZHENG等[154]制备了一种具有良好黏附性的壳聚糖-岩藻多糖的载药水凝胶贴片,ZHANG等[155]利用光触发S-亚硝基化偶联反应制备的一种薄而有弹黏性的可降解改性透明质酸凝胶,在保护黏膜伤口免受液体冲洗、抵抗口腔运动和摩擦的干扰方面展现出优越的性能,这些具有锚定作用的水凝胶辅料能较牢固地黏附在口腔黏膜上,实现药物控释的同时保证了说话、咀嚼等正常生理活动。
此外,健康口腔环境中的各类微生物群处于一种平衡状态,因此保护口腔微生态平衡也是研发口腔黏膜辅料所重视的必要条件。XIANG等[156]制备的一种具有自愈合能力并由紫外光触发黏附性的水凝胶贴剂,通过抗菌与抗氧化作用加速糖尿病患者口腔黏膜愈合进程。ZHAO等[157]制备的富勒烯醇可喷涂温控水凝胶,一方面清除辐射诱导的过量活性氧来抑制细胞凋亡,并上调抗氧化酶活性,促进黏膜上皮细胞的增殖和迁移,另一方面能维持口腔微生物群稳态。
生物材料在组织修复与再生中有着广泛的应用前景,但就目前研究来看,在OSF治疗方面的应用研究仍然较少,寻找适用OSF的特殊生物支架材料仍然需要更进一步的研究。
2.5.3 组织工程口腔黏膜 临床上用于修复口内伤口和软组织缺损的材料多源于自体组织移植,包括分层或全层皮肤移植、游离的口腔黏膜移植、局部皮瓣、带蒂远端皮瓣和微血管皮瓣等。虽然来自其他上皮组织的自体移植物仍是金标准,但存在供体部位损伤、组织可用性有限和整合较差等缺点[158]。因此,亟需可代替自体移植物来修复口腔黏膜缺损的替代方案。组织工程是解决口腔黏膜缺损的有前途的方案。
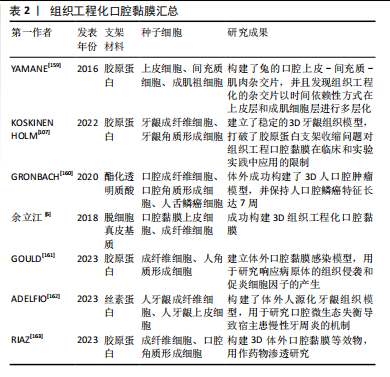
YAMANE等[159]用离体的上皮细胞、间充质细胞和成肌细胞祖细胞构建了兔的口腔上皮-间充质-肌肉杂交片,并且发现组织工程化的杂交片以时间依赖性方式在上皮层和成肌细胞层进行多层化。KOSKINEN HOLM等[107]将胶原凝胶与京尼蛋白/细胞松弛素D直接交联接种人永生化牙龈成纤维细胞,并在上表面接种人永生化牙龈角质形成细胞建立了稳定的3D牙龈组织模型,打破了胶原蛋白支架收缩问题对组织工程口腔黏膜在临床和实验实践中应用的限制。
组织工程口腔黏膜不仅能作为自身黏膜替代物,修复缺损组织,还能用于体外构建口腔相关疾病模型以及评估口腔材料生物相容性,有利于研究黏膜类疾病的发生机制[160]。通过对成纤维细胞与角质形成细胞分层培养,体外构建三维口腔黏膜复合体[6],不仅可移植到缺损部位促进皮肤组织愈合,还可作为体外模型分析白色念珠菌感染机制[161]、口腔微生物稳态[162]、口腔黏膜药物渗透作用等[163],有利于在体外对疾病发生发展与改进治疗方案进行更深入的研究。此外,较多的临床应用案例将组织工程化口腔黏膜用于重建人工尿道[164-166],而目前还未见有关组织工程化黏膜直接用于口腔黏膜修复的报道,可能是由于口腔内唾液、咀嚼、吞咽等特殊因素导致移植后的环境不稳定,限制了它的应用,见表2。
| [1] RAY JG, CHATTERJEE R, CHAUDHURI K. Oral submucous fibrosis: A global challenge. Rising incidence, risk factors, management, and research priorities. Periodontol 2000. 2019;80(1):200-212. [2] ARORA R, ADWANI D, NAPHADE M, et al. Malignant conversion of oral submucous fibrosis in surgically treated case. J Clin Diagn Res. 2014;8(10):ZD31-32. [3] GUO ZX, ZHANG Z, YAN JF, et al. A biomaterial-based therapy using a sodium hyaluronate/bioglass composite hydrogel for the treatment of oral submucous fibrosis. Acta Biomater. 2023;157:639-654. [4] 罗崇岱.高压氧对博来霉素诱导的口腔黏膜下纤维化模型E钙粘素表达的影响[D].长沙:中南大学,2012. [5] AVERINENI RK, SUNDERAJAN SG, MUTALIK S, et al. Development of mucoadhesive buccal films for the treatment of oral sub-mucous fibrosis: a preliminary study. Pharm Dev Technol. 2009;14(2):199-207. [6] 余立江,邵晓琳,龙笑,等.三维组织工程化口腔黏膜体外构建研究[J].中国实用口腔科杂志,2018,11(5):291-294. [7] KHEUR S, SANAP A, KHARAT A, et al. Hypothesizing the therapeutic potential of mesenchymal stem cells in oral submucous fibrosis. Med Hypotheses. 2020;144:110204. [8] SHAO Z, XU J, XU X, et al. Exosomes Derived from Human Adipose Mesenchymal Stem Cells Inhibits Fibrosis and Treats Oral Submucous Fibrosis via the miR-181a-5p/Smad2 Axis. Tissue Eng Regen Med. 2024;21(1):123-135. [9] BINNIE WH, CAWSON RA. A new ultrastructural finding in oral submucous fibrosis. Br J Dermatol. 1972;86(3):286-290. [10] PINDBORG JJ, CHAWLA TN, SRIVASTAVA AN, et al. Clinical aspects of oral submucous fibrosis. Acta Odontol Scand. 1964;22:679-691. [11] QIN X, NING Y, ZHOU L, et al. Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets. Int J Mol Sci. 2023;24(5):4992. [12] 张晶,代佳慧,代文婷,等.槟榔生物碱致癌性、致口腔黏膜下纤维化及兴奋作用等活性研究进展[J].食品与发酵工业,2023, 49(18):356-364. [13] ZHANG P, CHUA NQE, DANG S, et al. Molecular Mechanisms of Malignant Transformation of Oral Submucous Fibrosis by Different Betel Quid Constituents-Does Fibroblast Senescence Play a Role? Int J Mol Sci. 2022;23(3):1637. [14] ARORA S, SQUIER C. Areca nut trade, globalisation and its health impact: perspectives from India and South-east Asia. Perspect Public Health. 2019;139(1):44-48. [15] GUPTA AK, TULSYAN S, THAKUR N, et al. Chemistry, metabolism and pharmacology of carcinogenic alkaloids present in areca nut and factors affecting their concentration. Regul Toxicol Pharmacol. 2020;110:104548. [16] YANG SF, TSAI CH, CHANG YC. The upregulation of heat shock protein 47 expression in human buccal fibroblasts stimulated with arecoline. J Oral Pathol Med. 2008;37(4):206-210. [17] 禹洁,左巧娟,伍春华,等.FLG、分泌型卷曲相关蛋白1、转化生长因子β1在口腔黏膜下纤维化患者中的表达[J].北京口腔医学, 2022,30(6):398-401. [18] LEE SS, CHEN YJ, TSAI CH, et al. Elevated transglutaminase-2 expression mediates fibrosis in areca quid chewing-associated oral submucocal fibrosis via reactive oxygen species generation. Clin Oral Investig. 2016;20(5):1029-1034. [19] LIAO YW, YU CC, HSIEH CW, et al. Aberrantly downregulated FENDRR by arecoline elevates ROS and myofibroblast activation via mitigating the miR-214/MFN2 axis. Int J Biol Macromol. 2024;264(Pt 1):130504. [20] ILLEPERUMA RP, KIM DK, PARK YJ, et al. Areca nut exposure increases secretion of tumor-promoting cytokines in gingival fibroblasts that trigger DNA damage in oral keratinocytes. Int J Cancer. 2015;137(11): 2545-2557. [21] LIN KH, LIN CY, LIU CC, et al. Arecoline N-oxide: its mutagenicity and possible role as ultimate carcinogen in areca oral carcinogenesis. J Agric Food Chem. 2011;59(7):3420-3428. [22] KO AM, TU HP, KO YC. Systematic Review of Roles of Arecoline and Arecoline N-Oxide in Oral Cancer and Strategies to Block Carcinogenesis. Cells. 2023;12(8):1208. [23] SACHDEV PK, FREELAND-GRAVES J, BERETVAS SN, et al. Zinc, Copper, and Iron in Oral Submucous Fibrosis: A Meta-Analysis. Int J Dent. 2018; 2018:3472087. [24] HE XF, WANG H, TIAN Y, et al. Evaluation of Copper Levels in Dental Calculus of OSF Patients with Chewing Dried Areca-Nut Quids in Hunan Province of Mainland China. Biol Trace Elem Res. 2023;201(2):677-682. [25] AYINAMPUDI BK, NARSIMHAN M. Salivary copper and zinc levels in oral pre-malignant and malignant lesions. J Oral Maxillofac Pathol. 2012;16(2):178-182. [26] ARAKERI G, VISHAL RAO US, PATIL S, et al. Evaluation of fluoride levels in areca nut, tobacco, and commercial smokeless tobacco products: a pilot study. Br J Oral Maxillofac Surg. 2024;62(1):76-82. [27] WANG L, TANG Z. Immunopathogenesis of oral submucous fibrosis by chewing the areca nut. J Leukoc Biol. 2022;111(2):469-476. [28] WANG SY, ZHANG SJ, MENG HF, et al. DPSCs regulate epithelial-T cell interactions in oral submucous fibrosis. Stem Cell Res Ther. 2024; 15(1):113. [29] LIU S, LIU Z, SHAN Z, et al. Skewed Th17/Treg balance during progression and malignant transformation of oral submucous fibrosis. Oral Dis. 2022; 28(8):2119-2130. [30] WU D, LIU X, ZHOU T, et al. The expression of Th17/Treg in oral submucosal fibrosis carcinogenesis and the significance in the development of mucosal lesions. Cell Mol Biol (Noisy-le-grand). 2023; 69(9):84-88. [31] MOHAPATRA D, PANDA S, MOHANTY N, et al. Comparison of Immunohistochemical Markers in Oral Submucous Fibrosis and Oral Submucous Fibrosis Transformed to Oral Squamous Cell Carcinoma-A Systematic Review and Meta-Analysis. Int J Mol Sci. 2023;24(14):11771. [32] XU Z, CHEN D, HU Y, et al. Anatomically distinct fibroblast subsets determine skin autoimmune patterns. Nature. 2022;601(7891): 118-124. [33] SUTHERLAND TE, DYER DP, ALLEN JE. The extracellular matrix and the immune system: A mutually dependent relationship. Science. 2023;379(6633):eabp8964. [34] ZHOU S, CHEN L, MASHRAH M, et al. Expression and promoter methylation of Wnt inhibitory factor-1 in the development of oral submucous fibrosis. Oncol Rep. 2015;34(5):2636-2642. [35] SUDHAKARAN A, HALLIKERI K, BABU B. p16 as an independent marker for detection of high-risk HPV in oral submucous fibrosis and oral squamous cell carcinoma. Indian J Pathol Microbiol. 2019;62(4):523-528. [36] KUNDU P, PANT I, JAIN R, et al. Genome-wide DNA methylation changes in oral submucous fibrosis. Oral Dis. 2022;28(4):1094-1103. [37] TAN Y, HUANG Y, GUO L, et al. HLA-DQB1 Allele Polymorphism Associated with Oral Submucous Fibrosis in Hunan, China. J Immunol Res. 2024;2024:8757860. [38] MANNAN A, BHINDER MA, SADIA H, et al. Association of Oral Submucous Fibrosis Risk with <em>GSTM1</em> and <em>GSTT1</em> Gene Polymorphisms. J Coll Physicians Surg Pak. 2024;34(3):296-301. [39] 宋子毅,杨超,张云龙,等.基于基因表达综合数据库芯片挖掘结合网络药理学与分子对接探讨芒果苷治疗口腔黏膜下纤维化的机制研究[J].华西口腔医学杂志,2024,42(4):444-451. [40] SHARMA M, HUNTER KD, FONSECA FP, et al. Emerging role of cellular senescence in the pathogenesis of oral submucous fibrosis and its malignant transformation. Head Neck. 2021;43(10):3153-3164. [41] PENG CY, LIAO YW, LU MY, et al. Positive Feedback Loop of SNAIL-IL-6 Mediates Myofibroblastic Differentiation Activity in Precancerous Oral Submucous Fibrosis. Cancers (Basel). 2020;12(6):1611. [42] PANT I, KUMAR N, KHAN I, et al. Role of Areca Nut Induced TGF-β and Epithelial-Mesenchymal Interaction in the Pathogenesis of Oral Submucous Fibrosis. PLoS One. 2015;10(6):e0129252. [43] WANG W, XIONG H, HU Z, et al. Experimental study on TGF-β1-mediated CD147 expression in oral submucous fibrosis. Oral Dis. 2018; 24(6):993-1000. [44] HU HH, CHEN DQ, WANG YN, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76-83. [45] XIE C, ZHONG L, FENG H, et al. Exosomal miR-17-5p derived from epithelial cells is involved in aberrant epithelium-fibroblast crosstalk and induces the development of oral submucosal fibrosis. Int J Oral Sci. 2024;16(1):48. [46] RAI A, PARVEEN S, SHREE P, et al. Salivary transforming growth factor beta in oral submucous fibrosis: A diagnostic and predictive marker. J Cancer Res Ther. 2024;20(1):275-280. [47] WANG Z, HAN Y, PENG Y, et al. Senescent epithelial cells remodel the microenvironment for the progression of oral submucous fibrosis through secreting TGF-β1. PeerJ. 2023;11:e15158. [48] ABDUL AZIZ SHAIKH S, DENNY EC, KUMARCHANDRA R, et al. Evaluation of salivary tumor necrosis factor α as a diagnostic biomarker in oral submucosal fibrosis and squamous cell carcinoma of the oral cavity and oropharynx: a cross sectional observational study. Front Oral Health. 2024;5:1375162. [49] PAPANDREOU I, CAIRNS RA, FONTANA L, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187-197. [50] LEE P, CHANDEL NS, SIMON MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268-283. [51] TSAI CH, LEE SS, CHANG YC. Hypoxic regulation of plasminogen activator inhibitor-1 expression in human buccal mucosa fibroblasts stimulated with arecoline. J Oral Pathol Med. 2015;44(9):669-673. [52] GAWRONSKA-KOZAK B, MACHCINSKA-ZIELINSKA S, WALENDZIK K, et al. Hypoxia and Foxn1 alter the proteomic signature of dermal fibroblasts to redirect scarless wound healing to scar-forming skin wound healing in Foxn1-/- mice. BMC Biol. 2024;22(1):193. [53] R K, CHANDRA A, JAIN T, et al. An enigmatic pathogenetic mechanism of hypoxia inducible factor - 1/2 alpha in the progression of fibrosis of oral submucous fibrosis and its malignant transformation: A systematic review and meta-analysis. Arch Oral Biol. 2024;162:105944. [54] YANG X, ZHAO H, LI R, et al. Stromal thrombospondin 1 suppresses angiogenesis in oral submucous fibrosis. Int J Oral Sci. 2024;16(1):17. [55] PANDIAR D, NAIR SK, BOLOGNA-MOLINA R, et al. Correlation between Vascularity and Advancing Histological Grades of Oral Submucous Fibrosis with a Plausible Role in Malignisation: Systematic review of a persisting matter of conflict. Sultan Qaboos Univ Med J. 2024;24(2): 152-160. [56] MINO-OKA A, IZAWA T, SHINOHARA T, et al. Roles of hypoxia inducible factor-1α in the temporomandibular joint. Arch Oral Biol. 2017;73: 274-281. [57] GUPTA SR, SHARMA A, GUPTA N, et al. Single nucleotide polymorphisms and serologic levels of hypoxia-inducible factor1 α and vascular endothelial growth factor are associated with increased risk of oral submucous fibrosis in gutka users among a North Indian population. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(5):557-564. [58] SHARMA E, TYAGI N, GUPTA V, et al. Role of angiogenesis in oral submucous fibrosis using vascular endothelial growth factor and CD34: An immunohistochemical study. Indian J Dent Res. 2019;30(5):755-762. [59] SENEVIRATHNA K, MAHAKAPUGE TAN, JAYAWARDANA NU, et al. Serum mRNA levels of cytokeratin-19 and vascular endothelial growth factor in oral squamous cell carcinoma and oral potentially malignant disorders using RT-PCR. BMC Oral Health. 2024;24(1):1062. [60] LIN F, XIAO T, WANG B, et al. Mechanisms and markers of malignant transformation of oral submucous fibrosis. Heliyon. 2023;10(1):e23314. [61] WANG J, JIANG C, LI N, et al. The circEPSTI1/mir-942-5p/LTBP2 axis regulates the progression of OSCC in the background of OSF via EMT and the PI3K/Akt/mTOR pathway. Cell Death Dis. 2020;11(8):682. [62] LING Z, CHENG B, TAO X. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities. Int J Cancer. 2021;148(7):1548-1561. [63] ZANG W, LIU J, GENG F, et al. Butyrate promotes oral squamous cell carcinoma cells migration, invasion and epithelial-mesenchymal transition. PeerJ. 2022;10:e12991. [64] XU HQ, GUO ZX, YAN JF, et al. Fibrotic Matrix Induces Mesenchymal Transformation of Epithelial Cells in Oral Submucous Fibrosis. Am J Pathol. 2023;193(9):1208-1222. [65] 赵国强,王佳婷,郑灵娥,等.上皮-间质转化在口腔黏膜下纤维性变中的作用[J].医学研究杂志,2017,46(3):111-115. [66] MIRO C, DI CICCO E, AMBROSIO R, et al. Thyroid hormone induces progression and invasiveness of squamous cell carcinomas by promoting a ZEB-1/E-cadherin switch. Nat Commun. 2019;10(1):5410. [67] XIE C, LI Z, HUA Y, et al. Identification of a BRAF/PA28γ/MEK1 signaling axis and its role in epithelial-mesenchymal transition in oral submucous fibrosis. Cell Death Dis. 2022;13(8):701. [68] ANTAR SA, ASHOUR NA, MARAWAN ME, et al. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int J Mol Sci. 2023; 24(4):4004. [69] WYNN TA, RAMALINGAM TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028-1040. [70] SADAKSHARAM J, MAHALINGAM S. Evaluation of Oral Pentoxifylline in the Management of Oral Submucous Fibrosis - An Ultrasonographic Study. Contemp Clin Dent. 2017;8(2):200-204. [71] SHIL M, GOSWAMI P, GAIKWAD TV, et al. Efficacy of Oral Colchicine and Intralesional Hyaluronidase with and without Ultrasound Therapy in the Management of Oral Submucous Fibrosis-A Comparative Study. J Pharm Bioallied Sci. 2024;16(Suppl 1):S586-S588. [72] 唐瞻贵,成雨熹.口腔黏膜下纤维性变临床诊治的研究进展[J].口腔医学研究,2022,38(8):705-709. [73] LEUNG YY, YAO HUI LL, et al. Colchicine--Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3): 341-350. [74] JAMES L, SHETTY A, RISHI D, et al. Management of Oral Submucous Fibrosis with Injection of Hyaluronidase and Dexamethasone in Grade III Oral Submucous Fibrosis: A Retrospective Study. J Int Oral Health. 2015;7(8):82-85. [75] TP B, T AG, VARGHESE M, et al. Evaluation of Therapeutic Efficacy of Different Treatment Modalities in Oral Submucous Fibrosis: A Comparative Study. J Contemp Dent Pract. 2019;20(3):390-394. [76] MEHTA CH, PALIWAL S, MUTTIGI MS, et al. Polyphenol-based targeted therapy for oral submucous fibrosis. Inflammopharmacology. 2023;31(5):2349-2368. [77] ARAKERI G, PATIL S, MADDUR N, et al. Long-term effectiveness of lycopene in the management of oral submucous fibrosis (OSMF): A 3-years follow-up study. J Oral Pathol Med. 2020;49(8):803-808. [78] GUPTA N, KALASKAR A, KALASKAR R. Efficacy of lycopene in management of Oral Submucous Fibrosis- A systematic review and meta-analysis. J Oral Biol Craniofac Res. 2020;10(4):690-697. [79] SARAN G, UMAPATHY D, MISRA N, et al. A comparative study to evaluate the efficacy of lycopene and curcumin in oral submucous fibrosis patients: A randomized clinical trial. Indian J Dent Res. 2018; 29(3):303-312. [80] 刘昊泽,张善林,马微,等.螺旋藻藻蓝蛋白对乙醇致氧化应激小鼠抗氧化能力的影响[J].中国生物制品学杂志,2023,36(11):1301-1305. [81] 佘琴,石忠峰.螺旋藻抗氧化活性研究进展[J].广东药学院学报, 2014, 30(2):249-252. [82] KANJANI V, ANNIGERI RG, REVANAPPA MM, et al. Efficacy of Spirulina along with Different Physiotherapeutic Modalities in the Management of Oral Submucous Fibrosis. Ann Maxillofac Surg. 2019;9(1):23-27. [83] RAI A, SHRIVASTAVA PK, KUMAR A, et al. Comparative effectiveness of medicinal interventions for oral submucous fibrosis: A network meta-analysis. J Stomatol Oral Maxillofac Surg. 2023;124(3):101423. [84] YADAV M, ARAVINDA K, SAXENA VS, et al. Comparison of curcumin with intralesional steroid injections in Oral Submucous Fibrosis - A randomized, open-label interventional study. J Oral Biol Craniofac Res. 2014;4(3):169-173. [85] 张姗姗.姜黄素对口腔黏膜下纤维性变SD大鼠模型抗纤维化作用及机制的研究[D].长沙:中南大学,2012. [86] ZHANG L, TAN J, LIU Y, et al. Curcumin relieves arecoline-induced oral submucous fibrosis via inhibiting the LTBP2/NF-κB axis. Oral Dis. 2024; 30(4):2314-2324. [87] PÉREZ-LEAL M, LANCIANO F, FLACCO N, et al. Antioxidant treatments in patients with oral submucous fibrosis: A systematic review. J Oral Pathol Med. 2024;53(1):31-41. [88] DAI JP, ZHU DX, SHENG JT, et al. Inhibition of Tanshinone IIA, salvianolic acid A and salvianolic acid B on Areca nut extract-induced oral submucous fibrosis in vitro. Molecules. 2015;20(4):6794-6807. [89] ZHENG L, GUAN ZJ, PAN WT, et al. Tanshinone Suppresses Arecoline-Induced Epithelial-Mesenchymal Transition in Oral Submucous Fibrosis by Epigenetically Reactivating the p53 Pathway. Oncol Res. 2018;26(3):483-494. [90] 杨博,唐瞻贵.丹参联合醋酸曲安奈德注射液治疗口腔黏膜下纤维化的Meta分析[J].中成药,2018,40(10):2165-2169. [91] AL-MAWERI SA, ASHRAF S, LINGAM AS, et al. Aloe vera in treatment of oral submucous fibrosis: A systematic review and meta-analysis. J Oral Pathol Med. 2019;48(2):99-107. [92] ANURADHA A, PATIL B, ASHA VR. Evaluation of efficacy of aloe vera in the treatment of oral submucous fibrosis - a clinical study. J Oral Pathol Med. 2017;46(1):50-55. [93] SINGH N, HEBBALE M, MHAPUSKAR A, et al. Effectiveness of Aloe Vera and Antioxidant along with Physiotherapy in the Management of Oral Submucous Fibrosis. J Contemp Dent Pract. 2016;17(1):78-84. [94] RE K, PATEL S, GANDHI J, et al. Clinical utility of hyperbaric oxygen therapy in dentistry. Med Gas Res. 2019;9(2):93-100. [95] HOPF HW, GIBSON JJ, ANGELES AP, et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13(6):558-564. [96] ALMZAIEL AJ, BILLINGTON R, SMERDON G, et al. Hyperbaric oxygen enhances neutrophil apoptosis and their clearance by monocyte-derived macrophages. Biochem Cell Biol. 2015;93(4):405-416. [97] CANNELLOTTO M, YASELLS GARCÍA A, LANDA MS. Hyperoxia: Effective Mechanism of Hyperbaric Treatment at Mild-Pressure. Int J Mol Sci. 2024;25(2):777. [98] VADEPALLY AK, SALAVADI RK, SINHA R. A comparative clinical study on physiotherapy outcomes with wooden tongue depressors versus Heister jaw opener in oral submucous fibrosis patients. J Oral Biol Craniofac Res. 2019;9(3):263-267. [99] 谌静,吴颖芳,彭解英,等.张口训练增加口腔黏膜下纤维性变患者的张口度[J].中南大学学报(医学版),2021,46(7):731-735. [100] KHOLAKIYA Y, JOSE A, RAWAT A, et al. Surgical management of oral submucous fibrosis with “Seagull-nasolabial flap” combined with short-term oral pentoxifylline for preventing relapse. J Stomatol Oral Maxillofac Surg. 2020;121(5):512-516. [101] LAVIK E, LANGER R. Tissue engineering: current state and perspectives. Appl Microbiol Biotechnol. 2004;65(1):1-8. [102] BERTHIAUME F, MAGUIRE TJ, YARMUSH ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403-430. [103] IZUMI K, FEINBERG SE. Skin and oral mucosal substitutes. Oral Maxillofac Surg Clin North Am. 2002;14(1):61-71. [104] SONG D, LI Z, SUN F, et al. Optimized administration of human embryonic stem cell-derived immunity-and-matrix regulatory cells for mouse lung injury and fibrosis. Stem Cell Res Ther. 2024;15(1):344. [105] YUAN Y, LI J, LU X, et al. Autophagy in hepatic progenitor cells modulates exosomal miRNAs to inhibit liver fibrosis in schistosomiasis. Front Med. 2024;18(3):538-557. [106] HSIAO YC, WANG IH, YANG TL. Fibrotic remodeling and tissue regeneration mechanisms define the therapeutic potential of human muscular progenitors. Bioeng Transl Med. 2022;8(2):e10439. [107] KOSKINEN HOLM C, QU C. Engineering a 3D In Vitro Model of Human Gingival Tissue Equivalent with Genipin/Cytochalasin D. Int J Mol Sci. 2022;23(13):7401. [108] JIANG W, XU J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53(1):e12712. [109] YANG X, LI Q, LIU W, et al. Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: from pathogenesis to treatment. Cell Mol Immunol. 2023;20(6):583-599. [110] YAO L, HU X, DAI K, et al. Mesenchymal stromal cells: promising treatment for liver cirrhosis. Stem Cell Res Ther. 2022;13(1):308. [111] WANG Y, HUANG B, JIN T, et al. Intestinal Fibrosis in Inflammatory Bowel Disease and the Prospects of Mesenchymal Stem Cell Therapy. Front Immunol. 2022;13:835005. [112] CHENG W, ZENG Y, WANG D. Stem cell-based therapy for pulmonary fibrosis. Stem Cell Res Ther. 2022;13(1):492. [113] LI Z, NIU S, GUO B, et al. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020;53(12):e12939. [114] TANG Q, LU B, HE J, et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. 2022; 280:121320. [115] ROCKEL JS, RABANI R, VISWANATHAN S. Anti-fibrotic mechanisms of exogenously-expanded mesenchymal stromal cells for fibrotic diseases. Semin Cell Dev Biol. 2020;101:87-103. [116] AGGARWAL S, PITTENGER MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4): 1815-1822. [117] HAN MM, HE XY, TANG L, et al. Nanoengineered mesenchymal stem cell therapy for pulmonary fibrosis in young and aged mice. Sci Adv. 2023;9(29):eadg5358. [118] XU JH, XU SQ, DING SL, et al. Bone marrow mesenchymal stem cells alleviate the formation of pathological scars in rats. Regen Ther. 2022; 20:86-94. [119] FANG F, HUANG RL, ZHENG Y, et al. Bone marrow derived mesenchymal stem cells inhibit the proliferative and profibrotic phenotype of hypertrophic scar fibroblasts and keloid fibroblasts through paracrine signaling. J Dermatol Sci. 2016;83(2):95-105. [120] LI Y, CHEN S, TAN J, et al. Combination therapy with DHA and BMSCs suppressed podocyte injury and attenuated renal fibrosis by modulating the TGF-β1/Smad pathway in MN mice. Ren Fail. 2023;45(1):2120821. [121] TSUJI K, KITAMURA S, WADA J. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Renal Diseases. Int J Mol Sci. 2020;21(3):756. [122] XIANG E, HAN B, ZHANG Q, et al. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res Ther. 2020;11(1):336. [123] LI Y, ZHANG W, GAO J, et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7(1):102. [124] KANG Y, SONG Y, LUO Y, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate experimental non-alcoholic steatohepatitis via Nrf2/NQO-1 pathway. Free Radic Biol Med. 2022; 192:25-36. [125] ELMAHDY NA, SOKAR SS, SALEM ML, et al. Anti-fibrotic potential of human umbilical cord mononuclear cells and mouse bone marrow cells in CCl4- induced liver fibrosis in mice. Biomed Pharmacother. 2017;89:1378-1386. [126] HE S, WANG Q, CHEN L, et al. miR-100a-5p-enriched exosomes derived from mesenchymal stem cells enhance the anti-oxidant effect in a Parkinson’s disease model via regulation of Nox4/ROS/Nrf2 signaling. J Transl Med. 2023;21(1):747. [127] REGMI S, RAUT PK, PATHAK S, et al. Enhanced viability and function of mesenchymal stromal cell spheroids is mediated via autophagy induction. Autophagy. 2021;17(10):2991-3010. [128] WANG X, ZHAO S, LAI J, et al. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice. Int J Mol Sci. 2021;23(1):99. [129] CAO L, SU H, SI M, et al. Tissue Engineering in Stomatology: A Review of Potential Approaches for Oral Disease Treatments. Front Bioeng Biotechnol. 2021;9:662418. [130] SHI Q, XIA Y, WU M, et al. Mi-BMSCs alleviate inflammation and fibrosis in CCl4-and TAA-induced liver cirrhosis by inhibiting TGF-β/Smad signaling. Mater Today Bio. 2024;25:100958. [131] 张思宇,马诗淇,王梦慈,等.皮肤组织工程支架及其材料在创面修复领域中的研究进展[J].协和医学杂志,2023,14(3):603-610. [132] 王忠朝,范丽苑,蔡炜,等.丝素蛋白支架修复颊黏膜缺损[J].中国组织工程研究,2016,20(12):1738-1744. [133] 蒋灿华,李超,石芳琼,等.异种脱细胞真皮基质修复膜在口腔黏膜下纤维性变手术治疗中的应用[J].上海口腔医学,2011,20(3): 273-277. [134] 程梦可,杨杜娟,刘佳.甲基丙烯酸酐改性明胶/经处理牙本质基质生物活性支架的制备及性能[J].中国组织工程研究,2024,28(22):3555-3560. [135] QI W, DONG N, WU L, et al. Promoting oral mucosal wound healing using a DCS-RuB2A2 hydrogel based on a photoreactive antibacterial and sustained release of BMSCs. Bioact Mater. 2022;23:53-68. [136] ZHANG X, HASANI-SADRABADI MM, ZARUBOVA J, et al. Immunomodulatory Microneedle Patch for Periodontal Tissue Regeneration. Matter. 2022;5(2):666-682. [137] MEHTA CH, VELAGACHERLA V, MANANDHAR S, et al. Development of Epigallocatechin 3-gallate-Loaded Hydrogel Nanocomposites for Oral Submucous Fibrosis. AAPS PharmSciTech. 2024;25(4):66. [138] CHENG X, YANG Y, LIAO Z, et al. Drug-loaded mucoadhesive microneedle patch for the treatment of oral submucous fibrosis. Front Bioeng Biotechnol. 2023;11:1251583. [139] ALTMAN AM, MATTHIAS N, YAN Y, et al. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials. 2008; 29(10):1431-1442. [140] LIANG L, CUI R, ZHONG S, et al. Analysis of the potential role of photocurable hydrogel in patient-derived glioblastoma organoid culture through RNA sequencing. Biomater Sci. 2022;10(17):4902-4914. [141] LI X, LI Y, YU C, et al. ROS-Responsive Janus Au/Mesoporous Silica Core/Shell Nanoparticles for Drug Delivery and Long-Term CT Imaging Tracking of MSCs in Pulmonary Fibrosis Treatment. ACS Nano. 2023; 17(7):6387-6399. [142] LV Y, YU C, LI X, et al. ROS-activatable nanocomposites for CT imaging tracking and antioxidative protection of mesenchymal stem cells in idiopathic pulmonary fibrosis therapy. J Control Release. 2023;357: 249-263. [143] BAO H, CHENG S, LI X, et al. Functional Au nanoparticles for engineering and long-term CT imaging tracking of mesenchymal stem cells in idiopathic pulmonary fibrosis treatment. Biomaterials. 2022;288:121731. [144] QU C, BAO Z, ZHANG X, et al. A thermosensitive RGD-modified hydroxybutyl chitosan hydrogel as a 3D scaffold for BMSCs culture on keloid treatment. Int J Biol Macromol. 2019;125:78-86. [145] YAO Y, YANG L, FENG LF, et al. IGF-1C domain-modified hydrogel enhanced the efficacy of stem cells in the treatment of AMI. Stem Cell Res Ther. 2020;11(1):136. [146] NIE M, KONG B, CHEN G, et al. MSCs-laden injectable self-healing hydrogel for systemic sclerosis treatment. Bioact Mater. 2022;17:369-378. [147] WU T, ZHANG X, LIU Y, et al. Wet adhesive hydrogel cardiac patch loaded with anti-oxidative, autophagy-regulating molecule capsules and MSCs for restoring infarcted myocardium. Bioact Mater. 2022;21: 20-31. [148] 李兢思,李俊欣,王思炜,等.浅析生物医用材料中伤口敷料的研究现状[J].生物医学工程与临床,2023,27(6):818-823. [149] ZHOU C, YANG Z, XUN X, et al. De novo strategy with engineering a multifunctional bacterial cellulose-based dressing for rapid healing of infected wounds. Bioact Mater. 2021;13:212-222. [150] YANG C, ZHANG Y, ZHANG X, et al. An injectable, self-healing, and antioxidant collagen- and hyaluronic acid-based hydrogel mediated with gallic acid and dopamine for wound repair. Carbohydr Polym. 2023;320:121231. [151] ZHAO ZY, LI PJ, XIE RS, et al. Biosynthesis of silver nanoparticle composites based on hesperidin and pectin and their synergistic antibacterial mechanism. Int J Biol Macromol. 2022;214:220-229. [152] XU L, ZHAO K, MIAO J, et al. High-strength and anti-bacterial BSA/carboxymethyl chitosan/silver nanoparticles/calcium alginate composite hydrogel membrane for efficient dye/salt separation. Int J Biol Macromol. 2022;220:267-279. [153] AN H, GU Z, ZHOU L, et al. Janus mucosal dressing with a tough and adhesive hydrogel based on synergistic effects of gelatin, polydopamine, and nano-clay. Acta Biomater. 2022;149:126-138. [154] ZHENG W, HAO Y, WANG D, et al. Preparation of triamcinolone acetonide-loaded chitosan/fucoidan hydrogel and its potential application as an oral mucosa patch. Carbohydr Polym. 2021;272: 118493. [155] ZHANG W, BAO B, JIANG F, et al. Promoting Oral Mucosal Wound Healing with a Hydrogel Adhesive Based on a Phototriggered S-Nitrosylation Coupling Reaction. Adv Mater. 2021;33(48):e2105667. [156] XIANG Y, PAN Z, QI X, et al. A cuttlefish ink nanoparticle-reinforced biopolymer hydrogel with robust adhesive and immunomodulatory features for treating oral ulcers in diabetes. Bioact Mater. 2024;39: 562-581. [157] ZHAO M, WANG C, JI C, et al. Ascidian-Inspired Temperature-Switchable Hydrogels with Antioxidant Fullerenols for Protecting Radiation-Induced Oral Mucositis and Maintaining the Homeostasis of Oral Microbiota. Small. 2023;19(27):e2206598. [158] IZUMI K, YORTCHAN W, AIZAWA Y, et al. Recent trends and perspectives in reconstruction and regeneration of intra/extra-oral wounds using tissue-engineered oral mucosa equivalents. Jpn Dent Sci Rev. 2023;59:365-374. [159] YAMANE S, HIGA K, UMEZAWA T, et al. Engineered three-dimensional rabbit oral epithelial-mesenchymal-muscular hybrid sheets. Int J Oral Sci. 2016;8(3):145-154. [160] GRONBACH L, JURMEISTER P, SCHÄFER-KORTING M, et al. Primary Extracellular Matrix Enables Long-Term Cultivation of Human Tumor Oral Mucosa Models. Front Bioeng Biotechnol. 2020;8:579896. [161] GOULD SJ, FOEY AD, SALIH VM. An organotypic oral mucosal infection model to study host-pathogen interactions. J Tissue Eng. 2023;14: 20417314231197310. [162] ADELFIO M, MARTIN-MOLDES Z, ERNDT-MARINO J, et al. Three-Dimensional Humanized Model of the Periodontal Gingival Pocket to Study Oral Microbiome. Adv Sci (Weinh). 2023;10(12):e2205473. [163] RIAZ A, GIDVALL S, PRGOMET Z, et al. Three-Dimensional Oral Mucosal Equivalents as Models for Transmucosal Drug Permeation Studies. Pharmaceutics. 2023;15(5):1513. [164] SCHWAB R, HELLER M, PFEIFER C, et al. Full-thickness tissue engineered oral mucosa for genitourinary reconstruction: A comparison of different collagen-based biodegradable membranes. J Biomed Mater Res B Appl Biomater. 2021;109(4):572-583. [165] 金衍丰,谢弘.口腔黏膜组织工程材料在尿道重建的研究进展[J].现代泌尿外科杂志,2017,22(4):312-315. [166] BARBAGLI G, HEIDENREICH A, ZUGOR V, et al. Urothelial or oral mucosa cells for tissue-engineered urethroplasty: A critical revision of the clinical outcome. Asian J Urol. 2020;7(1):18-23. |
| [1] | 吴妍廷, 李 宇, 廖金凤. 氧化镁纳米粒调控成骨与血管生成相关基因表达促进骨缺损愈合[J]. 中国组织工程研究, 2026, 30(8): 1885-1895. |
| [2] | 蒋星海, 宋玉林, 李德津, 邵建敏, 徐军志, 刘华凯, 吴应国, 沈岳辉, 冯思诚. 血管内皮生长因子165基因转染骨髓间充质干细胞构建血管化两亲性肽凝胶模块[J]. 中国组织工程研究, 2026, 30(8): 1903-1911. |
| [3] | 王奇飒, 卢雨征, 韩秀峰, 赵文玲, 石海涛, 徐 哲. 3D打印甲基丙烯酰化透明质酸/脱细胞皮肤水凝胶支架的细胞相容性[J]. 中国组织工程研究, 2026, 30(8): 1912-1920. |
| [4] | 潘鸿飞, 庄圳冰, 徐白云, 杨章阳, 林恺瑞, 詹冰晴, 蓝靖涵, 高 恒, 张南波, 林家煜. 不同浓度金诺芬抑制M1型巨噬细胞功能及修复糖尿病小鼠伤口的价值[J]. 中国组织工程研究, 2026, 30(6): 1390-1397. |
| [5] | 彭志伟, 陈 雷, 佟 磊. 木犀草素促进糖尿病小鼠创面愈合的作用与机制[J]. 中国组织工程研究, 2026, 30(6): 1398-1406. |
| [6] | 吕晓凡, 黄 懿, 丁留成. 糖尿病膀胱病的线粒体机制与干预治疗[J]. 中国组织工程研究, 2026, 30(6): 1508-1515. |
| [7] | 李林臻, 焦泓焯, 陈伟南, 张铭哲, 王建龙, 张君涛. 淫羊藿苷含药血清对脂多糖诱导人软骨细胞炎症损伤的影响[J]. 中国组织工程研究, 2026, 30(6): 1368-1374. |
| [8] | 陈 驹, 郑锦畅, 梁 振, 黄成硕, 林 颢, 曾 莉. β-石竹烯对小鼠膝骨关节炎的作用及机制[J]. 中国组织工程研究, 2026, 30(6): 1341-1347. |
| [9] | 温广伟, 甄颖豪, 郑泰铿, 周淑怡, 莫国业, 周腾鹏, 李海山, 赖以毅. 异银杏素对破骨细胞分化的影响和机制[J]. 中国组织工程研究, 2026, 30(6): 1348-1358. |
| [10] | 李郝静, 王 新, 宋成林, 张胜男, 陈云昕. 上斜方肌处体外冲击波与运动控制训练治疗慢性非特异性颈痛[J]. 中国组织工程研究, 2026, 30(5): 1162-1170. |
| [11] | 刘 煜, 雷森林, 周锦涛, 刘 辉, 李先辉. 有氧和抗阻运动改善肥胖相关认知障碍的作用机制[J]. 中国组织工程研究, 2026, 30(5): 1171-1183. |
| [12] | 王正业, 刘万林, 赵振群. miRNA在激素诱导股骨头坏死机制中的研究进展[J]. 中国组织工程研究, 2026, 30(5): 1207-1214. |
| [13] | 部洋洋, 宁新丽, 赵 琛. 关节腔注射治疗颞下颌关节骨关节炎:不同药物与多种联合治疗方案[J]. 中国组织工程研究, 2026, 30(5): 1215-1224. |
| [14] | 文 凡, 向 阳, 朱 欢, 庹艳芳, 李 锋. 运动干预改善2型糖尿病患者的微血管功能[J]. 中国组织工程研究, 2026, 30(5): 1225-1235. |
| [15] | 刘新月, 李春年, 李一卓, 徐世芳. 口腔牙槽骨缺损的再生修复[J]. 中国组织工程研究, 2026, 30(5): 1247-1259. |
文章对近年来OSF的发病因素、病理生理学改变以及不同治疗方式的优缺点进行了分析与总结,并论述了组织工程策略治疗OSF的意义与可行性,以期为临床研究和实践提供新思路。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 由第一作者在2024年10月进行检索。
1.1.2 检索文献时限 各数据库建库至2024年10月。
1.1.3 检索数据库 PubMed 数据库和中国知网。
1.1.4 检索词 中文检索词为“口腔黏膜下纤维化,纤维化,口腔黏膜,发病机制,治疗,组织工程,间充质干细胞,生物材料,生长因子,动物模型”;英文检索词 为“oral submucous fibrosis,fibrosis,oral mucosa,mechanism,treatment,tissue engineering,mesenchymal stem cells,biomaterial,growth factors,animal model”。
1.1.5 检索文献类型 研究原著、综述、述评和荟萃分析等。
1.1.6 手工检索情况 无。
1.1.7 检索策略 PubMed数据库和中国知网检索策略见图1。
1.1.8 检索文献量 共检索到文献2 654篇,其中中文文献300篇,英文文献2 354篇。
1.2 纳入与排除标准
1.2.1 纳入标准 ①有关OSF发病机制与治疗方式的文献;②组织工程三要素与OSF或纤维化相关的文献;③组织工程三要素与口腔黏膜修复相关的文献;④有关口腔黏膜下纤维化动物模型构建的文献;⑤同一领域中论点与证据可靠、内容详实的文献。
1.2.2 排除标准 ①与文章相关性较低或无关的文献;②内容陈旧、质量不高的文献;③重复性研究;④非中、英文文献;⑤无法获取全文的文献。
1.3 质量评估及筛选流程 通过剔除不符合纳入标准的文献,排除与研究目的相关性较低及内容重复、资料陈旧的文献,最终纳入中国知网18篇文献和 PubMed数据库148篇文献,共计166篇文献进行综述。
3.1 既往他人在该领域研究的贡献和存在的问题 此前大量研究从组织学、遗传学等角度揭示了OSF的发病机制与病理生理进程,对于OSF治疗方法的研究虽然取得了一定成果,但治疗方式仍然局限在药物治疗、手术治疗、物理疗法。现已有大量的研究推动了纤维化疾病的组织工程化治疗的发展,与其他器官的纤维化相比,OSF组织工程的研究范围和深度明显不足。在种子细胞方面,目前已有研究证实间充质干细胞具有抗氧化、抗纤维化以及免疫调节能力,并提出间充质干细胞治疗OSF的潜力。一些学者已将牙髓间充质干细胞、成肌祖细胞等移植疗法用于OSF动物模型,证明了间充质干细胞用于治疗OSF的科学性与可行性,但目前国内外将间充质干细胞疗法应用在OSF中的研究实例仍然较少,需要更多的研究在间充质干细胞治疗OSF方面进行完善与创新。此外,间充质干细胞治疗OSF的具体机制也需要进一步的研究。在支架材料方面,新兴的生物活性材料不断涌现,水凝胶、丝素蛋白、脱细胞外基质支架等是研究各类纤维化疾病治疗策略的热点,目前也有一些研究将支架材料应用于OSF治疗,有一部分研究已将它们应用到OSF动物模型进行体内验证,但多数研究仍处于体外实验阶段。在组织工程化口腔黏膜方面,目前的研究尚处于构建阶段,用于建立疾病的体外组织模型,然而将其应用到OSF动物模型体内的研究尚处于空白。由于口腔内的理化环境复杂,OSF动物模型的构建方式尚不成熟且耗时长,许多组织工程治疗方法的体内研究也受到限制。
3.2 作者综述区别于他人他篇的特点 该文章对OSF组织工程治疗策略的应用前景和研究进展的综述与现有文献相比呈现明显的差异:①在时效性方面,此综述报道了OSF组织工程中种子细胞、生物活性材料和组织工程化口腔黏膜的最新研究进展,对间充质干细胞在其他各器官纤维化治疗中具有的抗炎、抗氧化、免疫调节能力进行了总结,并分析了它在OSF治疗中的潜力。②在介绍支架材料时,与以往综述不同,该文章着重分析了用于其他各类纤维化疾病中的热门材料具有的特性,总结了适合用于口腔理化环境的材料,分析汇总了目前的支架材料在OSF修复中的应用。③文章除了介绍组织工程化口腔黏膜在伤口修复方面的作用,还重点介绍了建立体外口腔3D黏膜用于体外研究疾病发展机制方面的作用,并指出组织工程化口腔黏膜在OSF发病机制与治疗应用中受限制的原因,而以往综述鲜有报道。
3.3 综述的局限性 目前在OSF组织工程领域,尚缺乏对生长因子的报道,也缺乏将种子细胞、支架材料、生长因子三要素进行研究,大多只聚焦于种子细胞或支架材料方面。因此,该文未涉及对生长因子的分析以及三要素的综合应用,存在一定的局限性。此外,OSF动物模型的建立是研究组织工程治疗方法的基础,方法的有效性和安全性需要在动物模型中验证,为后续临床试验的开展及临床上的应用提供数据支持,但文章缺少对OSF疾病动物模型的总结归纳,存在一定的局限性,但该文章仍然能为OSF的组织工程治疗策略的研究提供一定的思路。
3.4 综述的重要意义与展望 综上所述,OSF发病机制复杂,涉及多种细胞及细胞因子。近年来的研究丰富了对OSF病理过程的认识。现有的临床治疗方法如药物治疗、手术及物理疗法等治疗效应单一,难以完全阻止病程的进展且不良反应较多,需要考虑综合治疗手段。综合调节口腔纤维化的病理生理过程,减少不良反应,提高患者依从性是治疗OSF的趋势。
随着组织工程与再生医学的发展,整合干细胞和生物材料的组织工程化黏膜在治疗口腔疾病中的潜力逐渐被发掘。很长一段时间以来OSF这一疾病并未受到充分的重视,对于OSF发病机制研究也在积累当中,加上OSF的动物模型耗时长、操作繁琐,口腔中特殊的理化环境也限制了它们在OSF中的研究。然而OSF与其他各类纤维化疾病的病理生理学改变相似。因此,从其他纤维化疾病的研究中获取思路和灵感,将组织工程治疗策略应用于OSF的治疗具有重要的意义。
在种子细胞种类选择与移植微环境创造方面,需要充分考虑OSF乏氧、高氧化应激以及慢性炎症等方面的病理微环境。一方面,可尽量选择获取来源广且不易造成二次创伤的细胞,例如骨髓间充质干细胞、成肌细胞、牙髓间充质干细胞等;另一方面,选择合适的移植方法并创造一个合适的移植环境,使细胞充分发挥作用也至关重要,例如可以将细胞负载于具有保护作用的支架材料上,并添加一些具有保护作用的药物或生长因子等,通过局部注射或建立微通道的方式递送细胞,保证细胞能充分发挥作用。此外,通过制备细胞外泌体来进行治疗,可打破物种限制,使得细胞来源范围更广,可控性更高。
对于生物材料的应用与开发方面,可探索并开发符合口腔特殊理化环境的材料,例如,具有黏附功能的贴剂、可建立递送微通道的微针、具有抵抗唾液冲刷侵蚀的疏水材料、具有药物控释功能的纳米材料等等。此外,也可充分考虑OSF的病理生理变化,设计智能响应微环境的生物活性材料或药物控释材料,对完善OSF的长期综合治疗具有重要意义。
组织工程化口腔黏膜的构建可应用于手术治疗后替代皮瓣移植,可以减少二次创伤。在体外构建组织工程化口腔黏膜可用于研究OSF发病机制与病理生理变化,还能够弥补OSF动物模型构建困难的问题。
随着研究者们的不断攻关与努力,OSF得到越来越多的关注与研究。受到以往对于其他纤维化疾病研究的启发,近年来终于出现了间充质干细胞与生物材料用于治疗OSF的研究,充分说明了它们应用于OSF治疗的可行性与科学性并且存在着巨大的开发潜力,相信接下来会有更多研究在OSF组织工程治疗策略中不断完善与创新,为综合治疗OSF提供新的解决方案。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
口腔黏膜下纤维化是一种容易恶变的慢性进行性疾病,它的发生与咀嚼槟榔、免疫调节异常、遗传易感等因素密切相关。目前传统治疗手段包括药物治疗、物理治疗、手术治疗等,但患者依从性低,治疗方式单一,导致效果不理想。近年来,组织工程治疗策略在其他各类纤维化疾病中的研究成为热点,给口腔黏膜下纤维化的治疗研究带来启发与灵感,为口腔黏膜下纤维化的综合治疗开辟了新路径。#br# 文章综述了口腔黏膜下纤维化发病机制以及治疗的最新进展,总结并分析间充质干细胞、生物支架材料、组织工程口腔黏膜在口腔黏膜下纤维化中的作用与研究进展,阐述了组织工程治疗策略对于实现口腔黏膜下纤维化的综合治疗和逆转纤维化的科学性与可行性,对于未来探索基于组织工程化的口腔黏膜下纤维化治疗方案,具有重要意义。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程#br#| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||