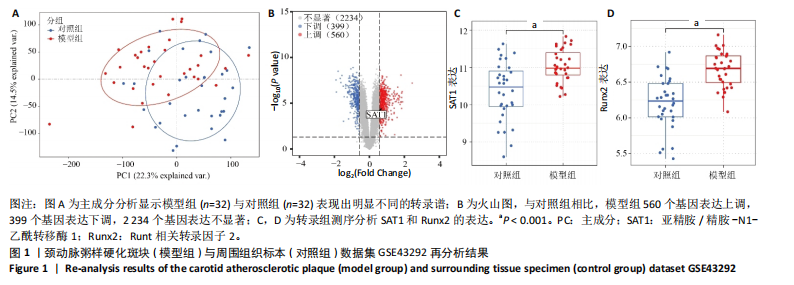
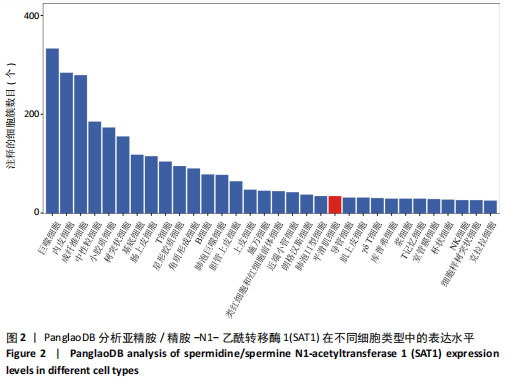
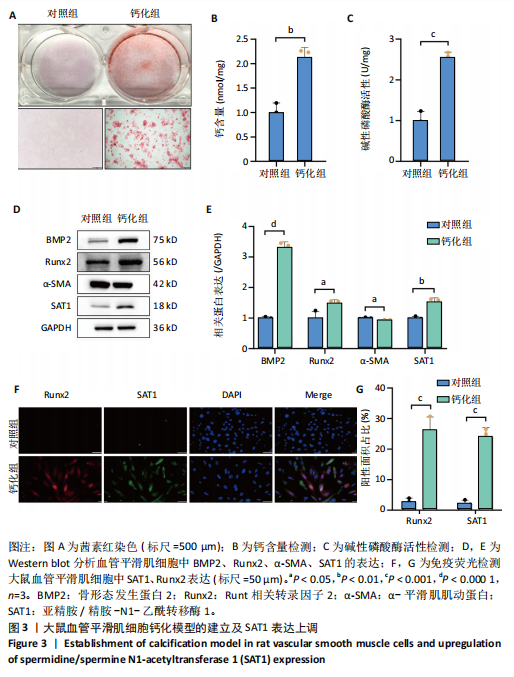
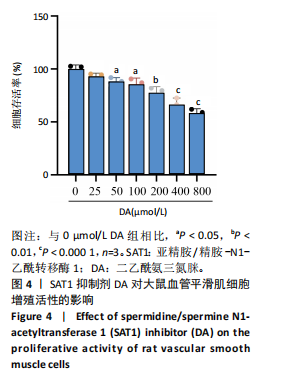
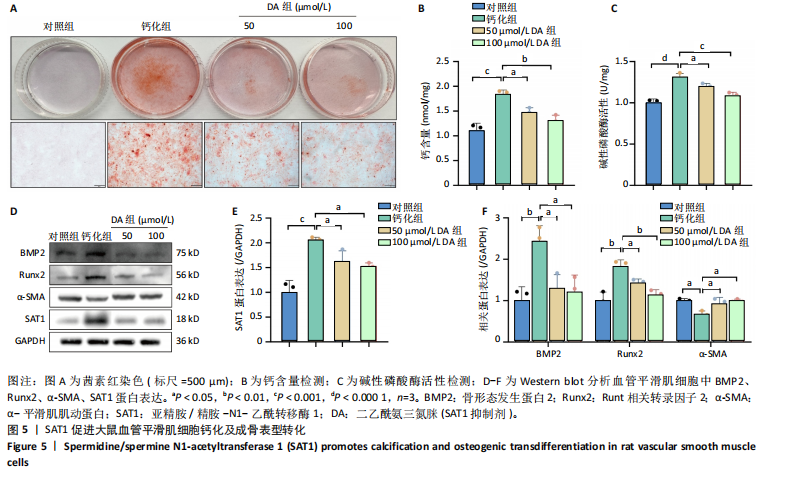
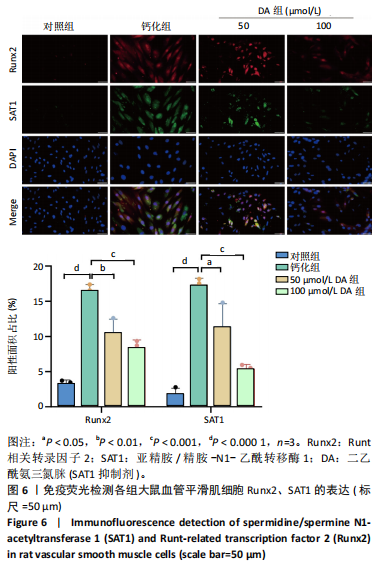
[1] VOELKL J, LANG F, ECKARDT KU, et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci. 2019;76(11):2077-2091.
[2] GUI Z, SHAO C, ZHAN Y, et al. Vascular calcification: High incidence sites, distribution, and detection. Cardiovasc Pathol. 2024;72:107667.
[3] KANG JH, KAWANO T, MURATA M, et al. Vascular calcification and cellular signaling pathways as potential therapeutic targets. Life Sci. 2024;336:122309.
[4] CHIANG HY, CHU PH, CHEN SC, et al. MFG-E8 promotes osteogenic transdifferentiation of smooth muscle cells and vascular calcification by regulating TGF-β1 signaling. Commun Biol. 2022;5(1):364.
[5] PAN W, JIE W, HUANG H. Vascular calcification: Molecular mechanisms and therapeutic interventions. MedComm (2020). 2023;4(1):e200.
[6] LIN X, SHAN SK, XU F, et al. The crosstalk between endothelial cells and vascular smooth muscle cells aggravates high phosphorus-induced arterial calcification. Cell Death Dis. 2022;13(7):650.
[7] LEE CC, CHUANG CC, CHEN CH, et al. In vitro and in vivo studies on exogenous polyamines and α-difluoromethylornithine to enhance bone formation and suppress osteoclast differentiation. Amino Acids. 2024;56(1):43.
[8] SHI M, GAN YJ, DAVIS TO, et al. Downregulation of the polyamine regulator spermidine/spermine N(1)-acetyltransferase by Epstein-Barr virus in a Burkitt’s lymphoma cell line. Virus Res. 2013;177(1):11-21.
[9] FANG W, XIE S, DENG W. Ferroptosis mechanisms and regulations in cardiovascular diseases in the past, present, and future. Cell Biol Toxicol. 2024;40(1):17.
[10] OU Y, WANG SJ, LI D, et al. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016;113(44): E6806-E6812.
[11] FRANZÉN O, BJÖRKEGREN JLM. alona: a web server for single-cell RNA-seq analysis. Bioinformatics. 2020;36(12):3910-3912.
[12] AYARI H, BRICCA G. Identification of two genes potentially associated in iron-heme homeostasis in human carotid plaque using microarray analysis. J Biosci. 2013;38(2):311-315.
[13] YANG S, ZENG Z, YUAN Q, et al. Vascular calcification: from the perspective of crosstalk. Mol Biomed. 2023;4(1):35.
[14] SIRACUSA C, CARINO A, CARABETTA N, et al. Mechanisms of Cardiovascular Calcification and Experimental Models: Impact of Vitamin K Antagonists. J Clin Med. 2024;13(5):1405.
[15] ZENG ZL, YUAN Q, ZU X, et al. Insights Into the Role of Mitochondria in Vascular Calcification. Front Cardiovasc Med. 2022;9:879752.
[16] SHANAHAN CM, CROUTHAMEL MH, KAPUSTIN A, et al. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011; 109(6):697-711.
[17] HOSHINO T, KHARAGHANI D, KOHNO S. Extracellular histones promote calcium phosphate-dependent calcification in mouse vascular smooth muscle cells. J Biochem. 2024;175(6):643-648.
[18] DONG Y, LIU Y, CHENG P, et al. Lower limb arterial calcification and its clinical relevance with peripheral arterial disease. Front Cardiovasc Med. 2023;10: 1271100.
[19] EL HADRI K, SMITH R, DUPLUS E, et al. Inflammation, Oxidative Stress, Senescence in Atherosclerosis: Thioredoxine-1 as an Emerging Therapeutic Target. Int J Mol Sci. 2021;23(1):77.
[20] SUTTON NR, MALHOTRA R, ST HILAIRE C, et al. Molecular Mechanisms of Vascular Health: Insights From Vascular Aging and Calcification. Arterioscler Thromb Vasc Biol. 2023;43(1):15-29.
[21] YANG H, LI Y, ZHU W, et al. SAT1/ALOX15 Signaling Pathway Is Involved in Ferroptosis After Skeletal Muscle Contusion. Int J Mol Sci. 2024;25(20):11317.
[22] BYON CH, JAVED A, DAI Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283(22):15319-15327.
[23] DURHAM AL, SPEER MY, SCATENA M, et al. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114(4):590-600.
[24] BENNION DM, HALTIGAN EA, IRWIN AJ, et al. Activation of the Neuroprotective Angiotensin-Converting Enzyme 2 in Rat Ischemic Stroke. Hypertension. 2015; 66(1):141-148.
[25] VEIT F, WEISSMANN N. Angiotensin-converting enzyme 2 activation for treatment of pulmonary hypertension. Am J Respir Crit Care Med. 2013;187(6):569-571.
[26] CHEN IC, LIN JY, LIU YC, et al. Angiotensin-Converting Enzyme 2 Activator Ameliorates Severe Pulmonary Hypertension in a Rat Model of Left Pneumonectomy Combined With VEGF Inhibition. Front Med (Lausanne). 2021;8:619133.
[27] QI Y, ZHANG J, COLE-JEFFREY CT, et al. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension. 2013;62(4):746-752.
[28] DE MARIA ML, ARAÚJO LD, FRAGA-SILVA RA, et al. Anti-hypertensive Effects of Diminazene Aceturate: An Angiotensin- Converting Enzyme 2 Activator in Rats. Protein Pept Lett. 2016;23(1):9-16.
[29] SHENOY V, GJYMISHKA A, JARAJAPU YP, et al. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013;187(6):648-657.
[30] VELKOSKA E, PATEL SK, GRIGGS K, et al. Short-term treatment with diminazene aceturate ameliorates the reduction in kidney ACE2 activity in rats with subtotal nephrectomy. PLoS One. 2015;10(3):e0118758.
[31] JIN Z, XU H, SUN X, et al. Targeting SAT1 prevents osteoporosis through promoting osteoclast apoptosis. Biomed Pharmacother. 2024;175:116732.
[32] LIU X, CHEN A, LIANG Q, et al. Spermidine inhibits vascular calcification in chronic kidney disease through modulation of SIRT1 signaling pathway. Aging Cell. 2021; 20(6):e13377.
[33] YATHINDRANATH V, SAFA N, SAJESH BV, et al. Spermidine/Spermine N1-Acetyltransferase 1 (SAT1)-A Potential Gene Target for Selective Sensitization of Glioblastoma Cells Using an Ionizable Lipid Nanoparticle to Deliver siRNA. Cancers (Basel). 2022;14(21):5179.
[34] NI YQ, LIU YS. New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging Dis. 2021;12(8):1948-1963.
[35] MANDAL S, MANDAL A, PARK MH. Depletion of the polyamines spermidine and spermine by overexpression of spermidine/spermine N¹-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem J. 2015;468(3):435-447.
[36] LI Y, JIE W, QI Y, et al. Inhibition of RIPK1 alleviating vascular smooth muscle cells osteogenic transdifferentiation via Runx2. iScience. 2023;27(2):108766.
[37] ATTA MG. A molecular target of vascular calcification in chronic kidney disease. J Clin Invest. 2022;132(1):e156257.
[38] ZHU S, CHEN W, MASSON A, et al. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024;10(1):71.
[39] AHN BY, JEONG Y, KIM S, et al. Cdon suppresses vascular smooth muscle calcification via repression of the Wnt/Runx2 Axis. Exp Mol Med. 2023;55(1):120-131.
[40] XU S, WANG F, MAI P, et al. Mechanism Analysis of Vascular Calcification Based on Fluid Dynamics. Diagnostics (Basel). 2023;13(16):2632. |