中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (25): 5454-5468.doi: 10.12307/2025.503
• 干细胞综述 stem cell review • 上一篇 下一篇
脑源性神经营养因子介导帕金森病的运动防治:作用与机制
雷森林1,谌晓安1,陈 平1,王兆锋1,2
- 1吉首大学体育科学学院,湖南省吉首市 416000;2北部湾大学体育学院,广西壮族自治区钦州市 535000
-
收稿日期:2024-03-22接受日期:2024-04-26出版日期:2025-09-08发布日期:2024-12-30 -
通讯作者:王兆锋,硕士,副教授,吉首大学体育科学学院,湖南省吉首市 416000;北部湾大学体育学院,广西壮族自治区钦州市 535000 -
作者简介:雷森林,男,1997年生,河南省人,吉首大学在读博士,主要从事运动慢病防治研究。 并列第一作者:谌晓安,女,1971年生,湖南省人,博士,教授,主要从事体质健康促进研究。 -
基金资助:湖南省自然科学基金(2021JJ30552),项目负责人:陈平;国家民族体育重点研究基地开放基金项目(MZTY2203),
项目负责人:陈平;湖南省教育厅科学研究重点项目(20A414),项目负责人:陈平
Exercise prevention and treatment of Parkinson’ s disease mediated by brain-derived neurotrophic factor: role and mechanism
Lei Senlin1, Chen Xiaoan1, Chen Ping1, Wang Zhaofeng1, 2
- 1College of Physical Education, Jishou University, Jishou 416000, Hunan Province, China; 2School of Physical Education, Beibu Gulf University, Qinzhou 535000, Guangxi Zhuang Autonomous Region, China
-
Received:2024-03-22Accepted:2024-04-26Online:2025-09-08Published:2024-12-30 -
Contact:Wang Zhaofeng, Master, Associate professor, College of Physical Education, Jishou University, Jishou 416000, Hunan Province, China; School of Physical Education, Beibu Gulf University, Qinzhou 535000, Guangxi Zhuang Autonomous Region, China -
About author:Lei Senlin, Doctoral candidate, College of Physical Education, Jishou University, Jishou 416000, Hunan Province, China; Chen Xiaoan, PhD, Professor, College of Physical Education, Jishou University, Jishou 416000, Hunan Province, China Lei Senlin and Chen Xiaoan contributed equally to this article. -
Supported by:Hunan Provincial Natural Science Foundation, No. 2021JJ30552 (to CP); Open Fund Project of National Key Research Base for Ethnic Sports, No. MZTY2203 (to CP); Key Scientific Research Project of Hunan Provincial Department of Education, No. 20A414 (to CP)
摘要:
文题释义:
帕金森病:是一种神经退行性疾病,主要发生于中老年人。帕金森病的主要病理特征为中脑黑质致密部多巴胺能神经元异常凋亡,以及错误折叠的α-突触核蛋白异常沉积,产生嗜酸性神经元包涵体——路易小体,同时还伴随剧烈的神经免疫炎症反应。脑源性神经影响因子:是继神经生长因子之后,在猪脑中纯化和表征神经源性蛋白后发现的第二个神经营养因子,其在中枢神经系统中调控神经系统稳态、促进神经元存活及突触可塑性、加强学习和记忆等方面发挥关键作用,因此被视为内源性“神经保护剂”。
摘要
背景:运动干预作为经济有效的非物理疗法,可有效上调脑源性神经营养因子表达进而防治帕金森病发生发展,但目前关于靶向脑源性神经营养因子的运动治疗策略在延缓帕金森病发生发展的潜在作用机制尚不明晰。
目的:以脑源性神经营养因子和帕金森病的关系为切入点,分析帕金森病病理状态下运动对脑源性神经营养因子表达的特异性调控作用及机制,梳理脑源性神经营养因子介导下不同运动方式对帕金森病的改善效果,并阐明靶向脑源性神经营养因子的运动治疗策略在防治帕金森病的潜在作用机制,旨在为运动防治帕金森病提供新的理论依据。
方法:以“帕金森病,脑源性神经营养因子,神经保护,多巴胺,神经元异常凋亡,神经炎症反应,突触可塑性,运动”等为中文检索词;以“Parkinson’s disease,BDNF,Neuroprotection,neuroinflammation,synaptic plasticity”等为英文检索词,分别检索中国知网、万方数据库、PubMed和Web of Science数据库,搜寻各数据库建库至2024年2月发表的所有研究文献,根据纳排标准共获得核心相关文献98篇。
结果与结论:①在帕金森病理背景下,运动可通过促进肌因子鸢尾素大量分泌,并降低色氨酸-犬尿氨酸代谢途径紊乱特异性调控脑源性神经营养因子表达。②有氧运动,尤其是特殊有氧运动(动物:旋转杆步行/人类:北欧健走)以及多模式运动可显著上调脑源性神经营养因子表达,进而改善帕金森病的运动症状,此外脑源性神经营养因子还介导身心运动(太极拳)对帕金森病患者认知障碍和睡眠障碍等非运动症状的有效调节。③运动诱导的高表达脑源性神经营养因子可能通过上调抗炎因子白细胞介素10、神经生长因子β和转化生长因子β表达,下调促炎因子肿瘤坏死因子α及白细胞介素1β表达,并抑制核转录因子κB信号通路表达降低小胶质细胞活性减轻神经炎症反应;增加酪氨酸羟化酶活性以促进多巴胺合成释放,并通过下调基质金属蛋白酶3及糖原合成酶激酶3β表达抑制α-突触核蛋白在丝氨酸129位点的磷酸化修饰,以防止神经异常凋亡;诱导突触效能的长时程增强发生,促进突触后致密区蛋白95及突触素大量表达以改善突触可塑性,发挥神经保护作。④鉴于脑源性神经营养因子在帕金森病发病进程及治疗中发挥重要作用,靶向脑源性神经营养因子的运动治疗策略将有助于推动帕金森病疾病“运动+药物”精准医疗的发展。但由于目前研究采用的运动处方较为单一,且研究焦点主要围绕运动症状而缺乏对非运动症状的考察,因此亟待学者采用更加统一和系统的运动处方,围绕非有氧运动类型对同一批帕金森病患者进行长期纵向跟踪研究,以此完善帕金森病运动防治领域研究的不足。
中图分类号:
引用本文
雷森林, 谌晓安, 陈 平, 王兆锋. 脑源性神经营养因子介导帕金森病的运动防治:作用与机制[J]. 中国组织工程研究, 2025, 29(25): 5454-5468.
Lei Senlin, Chen Xiaoan, Chen Ping, Wang Zhaofeng. Exercise prevention and treatment of Parkinson’ s disease mediated by brain-derived neurotrophic factor: role and mechanism[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5454-5468.
综上所述,BDNF在帕金森病治疗中的作用已经从最初的发现逐步过渡到运动干预策略的广泛研究,目前研究的焦点主要在探讨不同类型的运动对BDNF以及帕金森病改善效果之间的差异。现阶段的研究从帕金森病动物模型和患者临床研究层面证实了BDNF在帕金森病病理中的重要作用,并为运动通过上调BDNF表达进而治疗帕金森病的理论提供了一定的实践证明。
2.2 BDNF的分泌及表达 BDNF是继神经生长因子之后,由BARDE等[15]对猪脑中神经源性蛋白进行纯化和表征后第二个被发现的神经营养因子。尽管bdnf基因转录仅包含一个蛋白编码区,但却受多个启动子(外显子Ⅰ-Ⅸ)调控[16],其中外显子Ⅰ和Ⅳ是神经元依赖性BDNF转录表达的主要外显子,且对神经元活动高度敏感[17-18]。研究表明,在神经肽类和递质大量分泌、高频电刺激或θ脉冲刺激和节律性神经元电活动刺激下中枢脑组织,以及在肌纤维收缩、冷暴露和神经肌肉电刺激下外周骨骼肌组织是循环BDNF的主要分泌组织,一系列刺激源主要通过诱发外显子Ⅰ和Ⅳ依赖性bdnf基因表达完成BDNF蛋白合成,然后经囊泡运输主要作用于神经元细胞并发挥神经调控作用[19]。成熟BDNF蛋白合成经历pre-proBDNF和proBDNF两个前体的形态转化,而多种形式的BDNF发挥不同生物学功能受制于结合的直接受体:proBDNF优先结合p75 神经营养因子受体,可分别激活核转录因子κB参与神经元存活,以及激活c-Jun N-末端激酶信号通路启动凋亡联级反应加剧神经细胞凋亡[20]。相较而言,BDNF与原肌球蛋白相关激酶B结合则发挥有益的生理作用,BDNF使其同源二聚化并自磷酸化酪氨酸残基,并激活下游磷脂酶c-γ、磷脂酰肌醇-3激酶和丝裂原活化蛋白激酶3条主要信号通路表达,继而通过激活转录因子环磷酸腺苷反应元件结合蛋白表达并发挥调节神经元存活与分化、突触可塑性的积极作用[21-23]。需要提及的是,BDNF的神经保护作用与proBDNF的神经凋亡效果在正常生理状态下存在着动态平衡,BDNF/proBDNF的比值维持在稳定范围,但在病理状态下由于proBDNF蛋白裂解为成熟BDNF通路受阻,表现为BDNF/proBDNF平衡向proBDNF过多转变[24]。LUO等[25-26]通过系列研究证实,运动可通过发挥BDNF表达促进以及proBDNF表达抑制的双向调控效果上调BDNF/proBDNF比值,进而减轻疾病诱导的BDNF/proBDNF失衡并改善海马神经可塑性,提示运动对BDNF生物学功能稳态的调控具有重要作用。图3汇总了BDNF 的表达及其下游信号通路。
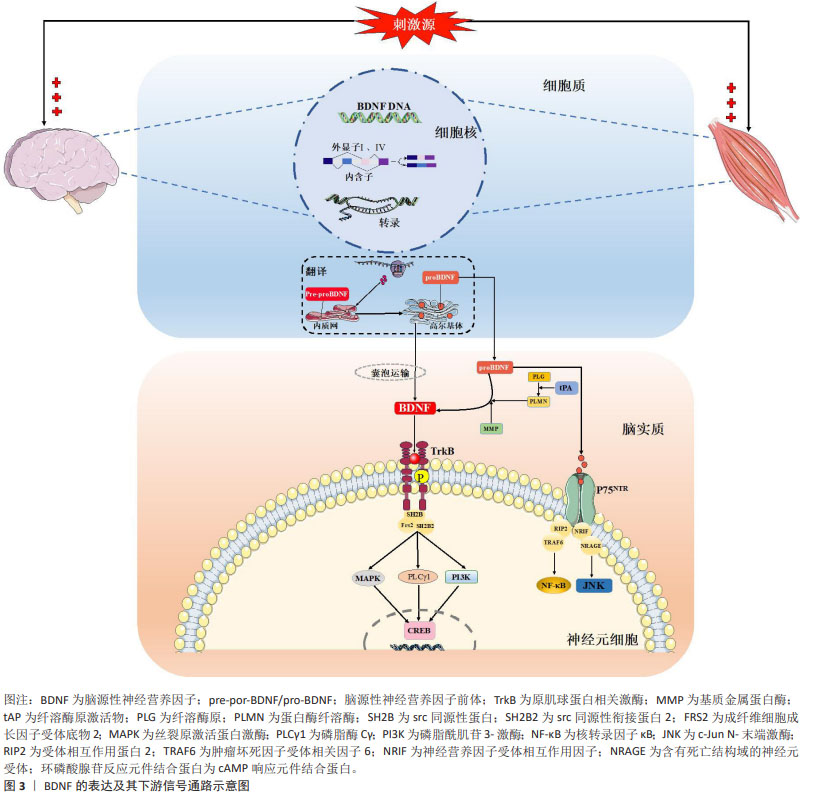
2.3 BDNF与帕金森病
2.3.1 BDNF在帕金森病中表达异常 有关帕金森病中BDNF异常表达的首次描述可追溯到20世纪末期,MOGI等[10]首次报道在帕金森病患者尸检过程中发现脑组织中BDNF蛋白浓度特异性降低,HOWELLS等[11]随后在帕金森病小鼠中脑黑质致密部也发现BDNF mRNA表达减少70%,且减少原因主要归结于多巴胺能神经元的丧失,同时神经元细胞表面BDNF mRNA表达密度与神经元大小呈显著正相关。进一步研究表明,帕金森病患者血清BDNF表达水平随纹状体中多巴胺转运体功能丧失而下降,提示BDNF的表达与帕金森病状态多巴胺能系统稳态失调有关[27]。SCALZO等[12]首次对帕金森病患者血清BDNF水平进行评估后发现,帕金森病患者血清循环BDNF水平显著低于健康对照组,尤其在帕金森病早期时的降低程度更为强烈,但随着疾病程度加深BDNF循环水平反而增加。通过多元回归分析进一步指出BDNF增加水平与反映帕金森病 症状严重程度的统一帕金森病评定量表Ⅰ/Ⅱ/Ⅲ得分呈显著正相关,这种帕金森病疾病早期BDNF表达显著下降,但随着疾病程度加剧其表达反而显著增加的矛盾现象可能是机体为了应对疾病造成的神经损伤所做出自我调节的代偿性生理反应[12]。随着研究深入,帕金森病患者脑脊液中BDNF水平变化情况逐渐进入学者研究范畴[28-30],研究先后均证实帕金森病患者脑脊液中BDNF表达水平的显著下调,且BDNF表达水平与帕金森病患者的认知能力呈显著正相关。现阶段学者们逐渐纳入proBDNF和BDNF两项结局指标继续对帕金森病患者血液中BDNF进行考察[31-32],YI等[32]研究发现,帕金森病患者血清proBDNF蛋白含量显著高于非帕金森病患者,而BDNF蛋白含量显著低于非帕金森病患者,且BDNF/proBDNF指标值也显著降低。研究提出,BDNF/proBDNF指标值可作为早期帕金森病发生和评价帕金森病进展的生物标志物,且诊断价值优于单独使用BDNF的评价效果。综上,帕金森病患者BDNF表达异常主要与中枢多巴胺能系统稳态失调有关。
2.3.2 BDNF是治疗帕金森病的潜在药物靶点 在帕金森病动物模型中,已有部分研究通过直接注射外源性BDNF、病毒载体基因传导、非病毒载体基因传导以及药理学诱导等技术增加BDNF在脑中的含量防治帕金森病发生[33-40]。神经毒素1-甲基-4-苯基-1,2,3,6-四氢吡啶和6-羟基多巴胺诱导的帕金森病模型在小鼠、大鼠和灵长类动物(非人类)中被广泛用于探索BDNF对帕金森病的治疗作用[41]。研究发现,在帕金森病猴模型脑脊液中注入外源性BDNF可显著降低黑质区域神经元细胞损伤[33],而对帕金森病小鼠以病毒载体基因转导BDNF后同样发现高表达BDNF可显著增加黑质及纹状体中多巴胺的水平[35]。随着研究深入,有学者进行神经肽多聚体纳米非病毒性载体传递的BDNF和多巴胺受体D3激动剂联合给药可强烈刺激黑质和纹状体中多巴胺能神经元活性的上调和数量的增多,进而促进黑质中多巴胺能神经元向纹状体的投射并恢复神经元树突棘数量,从而改善帕金森病诱发的肌僵直病症[39]。最新研究逐渐采用药理学方法进行深入探索,KHIDR等[40]对鱼滕酮诱导的帕金森病小鼠腹腔连续11 d给予福莫特罗(β2肾上腺素受体激动剂)治疗后发现,福莫特罗通过激活环磷酸腺苷反应元件结合蛋白/BDNF级联反应上调BDNF表达,激活神经存活信号轴表达减轻神经元凋亡,并维持多巴胺能神经元的功能完整性。另有研究证实[42],对1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的帕金森病小鼠进行连续3周的肌苷给药发现,肌苷通过诱发BDNF大量表达改善帕金森病小鼠黑质致密部和纹状体中多巴胺能神经元的丧失,显著改善小鼠运动功能障碍。然而上述神经保护效应在采用原肌球蛋白相关激酶B受体抑制剂或BDNF干扰RNA转染沉默后被显著抑制,提示在帕金森病防治过程中BDNF发挥着极其重要的作用。上述研究表明,外源性BDNF给药可有效防治帕金森病,因此是潜在的药物治疗靶点。
值得注意的是,目前在帕金森病患者的临床研究中未见采用外源性BDNF进行给药治疗的相关报道,一方面BDNF在体内半衰期短且生物利用度差,同时通过血脑屏障的渗透性很小,因此较难实现在神经中枢系统中的有效传递;另一方面由于酶降解所导致蛋白从脑循环中快速清除、大分子蛋白在非靶向组织中的蓄积以及诱发的剧烈免疫反应等各种客观原因的存在,造成BDNF可能无法精准作用于靶向组织[43-44]。因此从帕金森病动物模型得到有关BDNF表达的研究成果在应用于帕金森病患者中还需要漫长的临床研究得以确认。
2.4 帕金森病病理背景下运动对BDNF表达的特异性调控 运动作为有效的刺激形式,单次运动可能引能起机体BDNF表达的急性应答反应,而长期运动则可累积运动效益,诱导BDNF水平的持续改变,更为有效地维持神经中枢稳态以防治帕金森病。
2.4.1 运动对BDNF表达的调控作用 研究表明,急性运动能够暂时上调健康受试者血清BDNF水平,且作用效果可持续20-60 min[45-47],然而在帕金森病病理状态下运动对BDNF的调控作用效果研究之间存在争议。有研究发现,安排帕金森病患者以中等强度(60%-80%最大心率)进行36 min单次有氧运动干预无法促进血清BDNF水平增加,进一步提高运动强度(≥85%最大心率)进行16 min运动干预后仍无法提高BDNF表达[48]。与之相反,另有研究指出30 min中等强度有氧运动可显著提高帕金森病患者血清BDNF水平[31]。研究之间的矛盾结果可能源于帕金森病病理状态下多巴胺能神经元损伤及神经炎症诱发的BDNF表达异常,而单次急性运动不足以逆转BDNF异常表达现象;也可能归结于不同研究中运动方式和强度存在的差异造成运动刺激程度不同所致。
帕金森病病理状态下长期运动已被证实可有效上调BDNF表达水平。帕金森病动物模型研究显示,跑步机/跑轮有氧运动[13, 49-55]、以及负重爬梯抗阻运动均可显著提升小鼠脑组织中BDNF的表达水平[50]。在帕金森病患者临床研究也观察到,长期有氧及多模式运动干预均可显著上调BDNF表达,造成血清约13%,23%-35%的BDNF循环水平上升幅度[56-58]。然而当前研究普遍认为,运动类型并非BDNF表达调控的主要决定因素,而运动强度则显得更为关键[59]。有研究安排帕金森病患者分别以≥85%最大心率和60%-80%最大心率的两种强度进行持续12周的运动干预后发现,仅前者可显著提高患者血清BDNF水平[48]。其中可能的原因可归结于以下3点:①高强度运动诱发的乳酸聚集可通过激活组蛋白脱乙酰化酶表达上调腺苷酸活化蛋白激酶/过氧化物酶体增殖物激活受体γ共激活因子1α表达,进而促进BDNF 启动子Ⅰ的激活[60];②高强度运动造成能量代谢反应增强并在骨骼肌中表现出更高的脂肪氧化程度,而高表达BDNF可通过参与葡萄糖稳态和脂质代谢以满足能量代谢需求[61];③高强度运动可能导致局部肌肉缺氧和微损伤,激发神经内分泌反应并伴随炎症递质和生长因子的大量释放,而部分因子可在一定程度上参与对BDNF表达的调控[61]。
2.4.2 运动调控BDNF表达的作用机制 目前,学界关于运动调控BDNF表达的作用机制基本达成共识,其机制主要包括运动激活钙离子依赖的级联信号通路表达促进环磷酸腺苷反应元件结合蛋白磷酸化继而上调bdnf基因启动子Ⅳ转录表达、运动刺激脑血流量升高激活纤溶酶原激活物表达诱导pro-BDNF向成熟BDNF蛋白转化、以及运动诱发外周组织分泌大量运动因子促进BDNF表达3个方面[8]。而在帕金森病病理背景下,靶向BDNF的运动调节机制研究焦点主要围绕极具生物学价值的运动敏感肌肉因子——鸢尾素以及与帕金森病病症息息相关的色氨酸-犬尿氨酸代谢途径两个方面展开[62-63]。
BOSTROM等[64]首次证实鸢尾素属于骨骼肌分泌的肌因子,在过表达的过氧化物酶体增殖物激活受体γ共激活因子1α的转基因小鼠模型中观可察到肌肉细胞的大量释放,其主要由前体纤维连结蛋白Ⅲ型域包含蛋白5中裂解释并放于血液循环中。WRANN等[65]发现除外周分泌表达外,鸢尾素还在脑内不同区域表达,并在运动刺激下可对BDNF表达进行调控。此外,外周骨骼肌中鸢尾素的激活也参与对脑BDNF表达的调控表达:当对小鼠尾静脉注射携带纤维连结蛋白Ⅲ型域包含蛋白5的腺病毒后能够产生依赖于BDNF表达的突触可塑性改善效果,且作用效果与脑血管内注射结果相似,而阻断外周鸢尾素信号通路表达则会抑制突触效能的长时程增强效应发生,提示外周鸢尾素循环水平升高可能会造成中枢鸢尾素富集,进而参与神经系统的稳态调控[66]。目前研究表明,运动刺激外周鸢尾素穿过血脑屏障后可通过激活丝裂原活化蛋白激酶/胞外信号调节激酶和环磷酸腺苷酸/蛋白激酶A/环磷酸腺苷反应元件结合蛋白两条信号通路调控BDNF表达[66-67],但既往研究中鸢尾素发挥作用的受体未知,因此无法探知其发挥神经调控的具体机制。而关于帕金森病小鼠模型的新近研究证实,运动刺激下鸢尾素对BDNF表达的调控作用是通过整合素αV/β5介导的[55]:8周有氧运动可显著促进帕金森病小鼠骨骼肌、血清以及海马中的鸢尾素合成水平,过表达鸢尾素继而结合海马神经元上整合素αV/β5激活环磷酸腺苷反应元件结合蛋白并诱导BDNF表达,从而发挥减少多巴胺能神经元损伤、改善认知障碍的直接保护作用。此外,鸢尾素还可发挥间接神经保护作用:通过增强整合素αV/β5与多巴胺能神经元细胞表面抗原CD90之间相互作用,进而维持黑质中多巴胺能神经元向纹状体投射,提示运动刺激下鸢尾素的神经保护作用并非仅局限于BDNF调控方面,其对多巴胺能神经元的多重影响机制也具有较高的研究价值。
色氨酸-犬尿氨酸代谢途径是机体色氨酸的核心分解代谢途径,色氨酸可通过2种吲哚胺2,3-双加氧酶亚型氧化为中间代谢产物犬尿氨酸[68-69],后者根据细胞类型的差异,分别代谢为以喹啉酸为主的神经毒性物质和以犬尿喹啉酸为主的神经保护性产物。研究表明,帕金森病发生过程会伴随色氨酸-犬尿氨酸代谢途径中代谢产物和生物酶表达紊乱而失衡,表现为喹啉酸/犬尿喹啉酸平衡向喹啉酸过多转变[63]。而在外周肌细胞和中枢星形胶质细胞中发现,犬尿氨酸可通过犬尿氨酸氨基转移酶催化生成犬尿氨酸,犬尿氨酸一方面阻断喹啉酸引发的兴奋性毒性,另一方面清除活性氧簇、活性氮簇自由基以发挥神经保护作用[70]。AGUDELOD等[71]研究指出,8周跑轮运动干预可通过上调过氧化物酶体增殖物激活受体γ共激活因子1α表达显著提升抑郁模型小鼠机体内犬尿氨酸氨基转移酶活性,并诱导外周犬尿氨酸转化为犬尿喹啉酸,继而减轻色氨酸-犬尿氨酸代谢途径紊乱程度并减少犬尿氨酸聚集,以保护大脑免受抑郁压力诱导的神经损伤发生,与此同时BDNF表达的抑制作用也被显著改善。在帕金森病小鼠模型也发现,色氨酸-犬尿氨酸代谢途径紊乱可造成神经性毒素中间产物大量分泌,同时血清和脑组织中BDNF表达均显著下调,而在实施吲哚胺2,3-双加氧酶抑制剂干预后则发现神经毒素表达水平降低而BDNF表达水平增加,继而抑制氧化应激和神经炎症反应以降低神经元损失和凋亡程度,从而有效缓解帕金森病小鼠运动功能障碍,提示色氨酸-犬尿氨酸代谢途径对BDNF表达可能存在重要调控作用[72]。此外IERACI等[73]研究表明,纯合子敲入BDNF Met/Met(BDNF基因多态性)小鼠表现出色氨酸-犬尿氨酸代谢途径的过度激活,血浆及海马中吲哚胺2,3-双加氧酶亚型1和犬尿氨酸氨基转移酶2蛋白mRNA显著增加,但犬尿氨酸氨基转移酶1蛋白mRNA表达显著降低,且4周有氧运动无法改善色氨酸-犬尿氨酸代谢途径异常紊乱现象,同时焦虑、抑郁及认知缺陷等病症也持续发生。与之相反,运动则可有效改善BDNFval/val野生型小鼠的海马依赖性记忆功能,这可能与BDNF Met/Met小鼠BDNF蛋白表达和功能缺失有关[74]。上述研究结果既揭示色氨酸-犬尿氨酸代谢途径中的代谢产物参与BDNF的水平和功能的调控,也表明BDNF可直接作用于色氨酸-犬尿氨酸代谢途径的正常表达,两者之间可能存在双向调控作用。
2.5 BDNF介导下不同运动方式对帕金森病的改善效果
2.5.1 有氧运动 现有研究表明,靶向BDNF改善帕金森病的有效运动治疗策略普遍采用有氧运动。帕金森病动物模型研究发现,采用有氧(跑步机或跑轮)干预方式是早期研究主流[13, 49-55],研究结果证实4-10周的有氧运动可显著提升小鼠中枢BDNF表达水平,并有效防治帕金森病小鼠病症恶化,主要体现在运动协调和平衡能力的显著提高(旋转杆测试中旋转时间、速度和次数显著减少、开放场活动显著增加)和学习记忆能力的显著提升(水迷宫测试中潜伏期显著缩短、平台停留时间显著延长、路径长度显著减少) [13, 50-51, 55, 75]。帕金森病临床研究结果与之相似,当帕金森病患者以≤60%最大心率的强度进行4周跑步机有氧运动(60 min/次,5次/周)干预后发现患者血清BDNF水平显著增加,并显著降低统一帕金森病评定量表Ⅱ/Ⅲ量表评分,同时显著提高6 min步行测试成绩及平衡能力[56]。随着研究的深入,特殊有氧运动方式逐渐被学者纳入研究范畴。LEEM研究团队以更符合小鼠行为特征的技能学习训练-旋转杆步行训练进行系列研究[76-77]。前期研究表明,4周旋转杆步行训练促进腺苷酸活化蛋白激酶磷酸化以诱导黑质及纹状体中BDNF大量表达,进而显著增加黑质向纹状体神经元投射以改善神经元分化缺陷,同时旋转杆步行训练更有利于维持负责自动运动控制的皮质-基底神经节回路正常生理功能[76]。进一步研究证实,旋转杆步行训练还可通过抑制小胶质细胞的激活减轻神经炎症反应,说明旋转杆步行训练区别于传统有氧训练能够产生更佳的神经保护效益,采用运动技能学习训练可能更利于减轻帕金森病运动症状[77]。与帕金森病动物模型研究结果类似,HARRO等[7]安排帕金森病患者以主观疲劳感觉4-6/7-8级的中高运动强度完成北欧健走特殊有氧训练(每周两三次、持续6周)后,不仅发现患者血清BDNF水平显著增加且统一帕金森病评定量表评分值显著降低,还观察到北欧健走训练相较于传统有氧训练在6/10 min步行测试、起立行走测试中展现更佳的改善效果,这得益于北欧健走训练特殊的训练模式,健走时要求患者使用特殊手杖在各种地形中进行:这既通过大幅度摆臂增加上肢参与度以提高四肢协调能力,又通过不同地形的特异性有针对性地提高躯干协调稳定性以减少步态功能障碍。然而,围绕帕金森病患者进行有氧运动干预的部分研究存在一定争议:SOKE等[78]安排中期帕金森病患者以60%-80%最大摄氧量完成8周跑步机有氧运动(30 min/次、3次/周)后却发现血清循环BDNF水平无显著变化,同时用于评估帕金森病患者运动功能的统一帕金森病评定量表Ⅲ评分也无显著变化,这与以往研究结果相反[7, 56]。
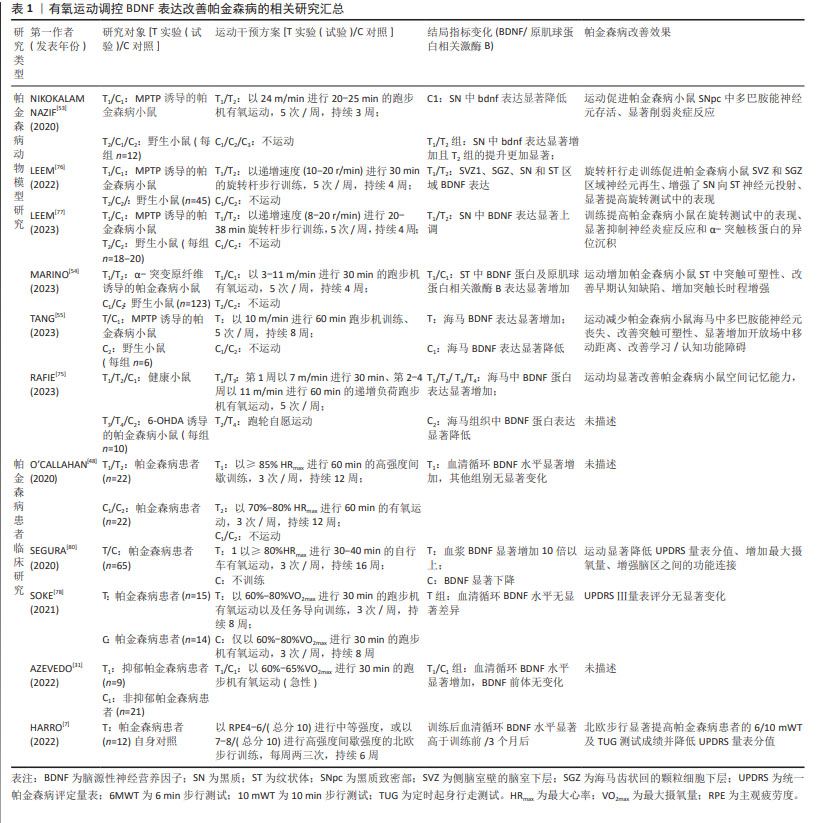
对不同研究中帕金森病患者的疾病严重程度进行比较分析发现,FRAZZITTA等[56]在研究中纳入患者主要处于霍恩-雅尔分期量表1.0-1.5等级,而SOKE等[78]的研究对象中93.1%处于2-3等级,循证医学结果显示,帕金森病患者血清BDNF水平显著低于健康人(MD= -2.99 ng/mL),且霍恩-雅尔分期量表等级越高(疾病程度越严重)BDNF表达越异常,提示BDNF介导下有氧运动对帕金森病的防治效果可能受帕金森病疾病严重程度影响[79]。表1汇总了近5年有氧运动调控BDNF表达改善帕金森病的相关研究。
2.5.2 多模式运动 需要提及的是,尽管TUON等[50]在帕金森病动物模型研究中发现,持续8周,每周进行三四次的50 min递增负荷(50%-100%体质量)爬梯抗阻运动可显著提高帕金森病小鼠纹状体和海马中BDNF表达水平,并减轻帕金森病小鼠抑郁样行为和旋转行为,但目前关于单一抗阻运动对BDNF表达调控作用的临床研究鲜见报道。现阶段以抗阻运动为主体,同时包含有氧、平衡以及柔韧运动等多种方式的多模式运动干预方式逐渐成为运动防治帕金森病的主流方式。由于多模式运动通常采用2种或2种以上类型混合的运动方式,因此其运动环境富集和类型的多样性可从多个维度(力量、协调、耐力、平衡和认知等)延缓帕金森病发生发展[81-82]。研究表明,当安排社区帕金森病患者以≥13主观疲劳感觉的强度进行长达8个月多模式运动训练(60 min/次,5次/周)后发现,多模式运动可显著上调帕金森病患者血清BDNF水平[81],并改善患者的步行能力(+5%)、功能活动能力(+11%)、下肢力量(+15%)、双侧握力(9%)以及认知功能。而LANDERS等[82]招募帕金森病患者进行8周多模式运动干预(90 min /次,3次/周)后也发现BDNF水平显著增加,该研究对帕金森病患者训练前后平衡、耐力/疲劳、活动能力及力量表现4个评价体系共31个评价指标进一步分析发现,高强度的多模式运动可显著改善7项指标(平衡评估系统测试、身体活动问卷、统一帕金森病评定量表Ⅲ、6 min步行测试、帕金森病疲劳量表、起立测试),但中等强度多模式运动仅改善3项指标(身体活动问卷、6 min步行测试和帕金森病疲劳量表),同时帕金森病患者对高强度多模式运动的依从性、不良事件发生率均与低强度运动无显著差异,提示高强度多模式运动可作为BDNF介导下帕金森病治疗更为有效的运动疗法。
然而另有研究发现,安排帕金森病患者以主观疲劳度≥13分/(总分20分)的运动强度进行8周多模式运动干预,但每周仅进行2次60 min的运动训练,虽然研究结果也证实运动显著提高患者定时起身行走测试和6 min步行测试成绩,并显著降低抑郁症状,但未能改善认知功能且血清BDNF水平也无显著影响[83]。从方法学角度分析发现,该研究的训练频率较低,每周仅进行2次,而大部分研究所采取的多模式运动频率均维持在3次及以上,提示运动频率也可能会影响多模式运动对BDNF调控作用以及帕金森病的治疗效果。
综上所述,高强度、多频率的多模式运动干预方式可能更有利于上调BDNF表达,进而更为有效地延缓帕金森病发生发展。
2.5.3 平衡运动 随着疾病发生发展帕金森病患者的运动症状逐渐加剧,平衡能力的下降尤为显著。而平衡运动则更具针对性地通过改善患者平衡功能障碍和姿态不稳定性,在一定程度上降低跌倒风险以预防患者残疾[84]。SZYMURA等[85]研究发现,安排帕金森病患者以60%-70%最大心率进行3次/周、为期12周的平衡训练可显著提高受试者血清循环BDNF水平,并显著提高平衡与步态量表评分值,且平衡训练可通过降低减轻患者神经炎症反应,诱导神经保护机制发生。但该研究仅发现平衡与步态评分值与抗炎因子白细胞介素10浓度变化呈正相关,与BDNF之间并无显著相关性。然而,进一步的随机、双盲研究结果却显示,每周2次、持续10周的平衡训练不仅无法提高帕金森病患者血清循环BDNF水平,且对患者平衡、步态以及执行功能等方面均未产生任何积极影响[86]。其可能的原因一方面源于该研究采用更为规范的随机、双盲试验方法,在一定程度上避免了试验偏差,提高了结果的客观性和规范性;另一方面相较于以往研究该研究采用的平衡运动干预频率及周期均较短,可能造成运动刺激程度较小无法诱发机体产生适应性变化,最终导致患者平衡功能障碍无法改善。
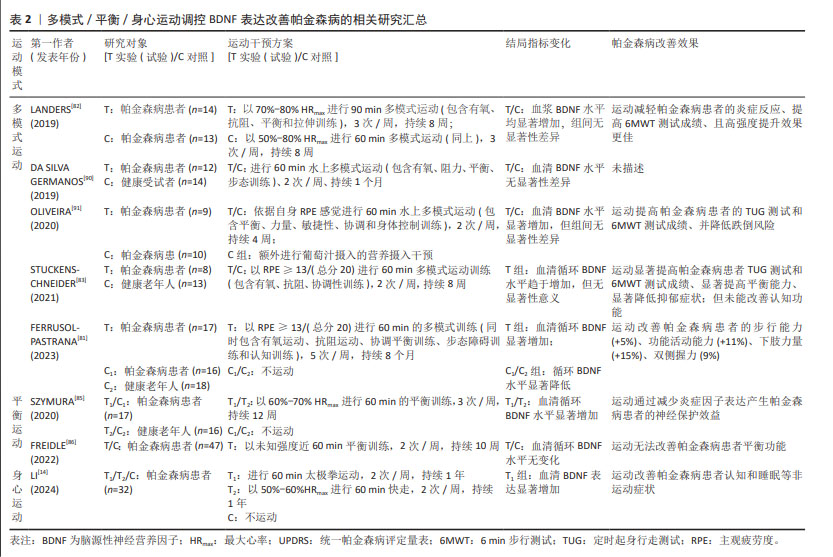
2.5.4 身心运动 在帕金森病的病理过程中,除典型的运动症状外,患者还普遍发生一系列精神行为异常、认知障碍以及睡眠障碍等非运动症状,且持续的心理挫败和焦虑会严重降低患者生活质量[87]。研究发现,瑜伽、太极拳、气功等身心运动通过增强身体感知与行为的协调性以及提升身体自我意识,有效地促进了心理健康和注意力集中,因此在缓解帕金森病患者的非运动症状方面显示出潜在的治疗价值[14]。KWOD等[88]研究发现,帕金森病患者对家庭式正念瑜伽身心训练模式的依从性极高,且瑜伽训练能够显著改善帕金森病患者的平衡功能、统一帕金森病评定量表和心理健康水平。然而目前大多数身心运动干预研究并未充分考量血液生化指标如细胞因子水平的变化情况。但在最新研究中,LI研究团队[14]提供了新的研究视角,证实了持续一年的太极拳运动可显著上调帕金森病患者血清BDNF水平,改善帕金森病认知评分量表得分并显著降低患者疲劳度量表成绩;且与快走运动相比,太极拳在改善量表得分方面展现出更显著的优势,这表明身心整合运动在缓解帕金森病非运动症状方面可能更具有优势。进一步相关性分析表明,上调的BDNF水平与帕金森病认知评分量表得分的改善程度,以及下调的嗜酸粒细胞趋化因子水平之间呈显著相关性,嗜酸粒细胞趋化因子作为神经炎症反应的参与者,其过表达与多巴胺神经元的凋亡有关[89]。研究提示身心整合运动可能通过调节BDNF的表达来抑制神经炎症,从而减轻帕金森病的非运动症状。这一新近发现不仅为靶向BDNF的帕金森病运动治疗策略提供了新视角,而且强调了在帕金森病的综合治疗中身心运动的重要性和潜在的神经保护作用[90-91]。表2汇总了近5年多模式/平衡/身心运动调控BDNF表达改善帕金森病的相关研究。
2.6 BDNF介导运动防治帕金森病的潜在作用机制
2.6.1 BDNF介导运动减轻帕金森病中神经炎症反应 剧烈神经炎症反应是神经中枢损伤的重要标志,也是诱发帕金森病的主要诱因[77]。通常促炎因子的过表达与抗炎因子的低表达所产生的失衡是炎症反应加剧的主要特征。衰老动物模型研究指出,长期运动可通过激活BDNF/原肌球蛋白相关激酶B信号通路表达抑制小鼠中枢神经炎症反应发生,但当对脑室内注射携带原肌球蛋白相关激酶B的慢病毒干预后则会消除运动对小鼠黑质和纹状体中神经炎症的抑制效果,导致核转录因子κB磷酸化表达水平增加并诱导促炎因子肿瘤坏死因子α和白细胞介素6大量分泌表达[92],说明BDNF/原肌球蛋白相关激酶B信号通路在运动抑制炎症反应中扮演重要角色。帕金森病动物模型研究证实,短期(3周)或长期(8周)有氧运动干预均可显著诱导帕金森病小鼠黑质中BDNF大量分泌[52-53],继而上调抗炎因子白细胞介素10表达并下调促炎因子白细胞介素1β、肿瘤坏死因子α的表达,说明BDNF可介导运动对帕金森病疾病状态下的抗炎/促炎失衡状态的有效调节。LEEM等[77]进一步研究发现,旋转杆步行训练上调BDNF表达后还可通过下调核转录因子κB信号通路表达抑制下游促炎因子肿瘤坏死因子α及白细胞介素1β表达,以此达到防止神经元损伤的目的。帕金森病患者临床研究证据也表明,平衡训练可上调帕金森病患者血清循环BDNF水平,并诱导抗炎细胞因子白细胞介素10、神经生长因子β以及转化生长因子β表达显著升高,以及促炎细胞因子肿瘤坏死因子α表达显著减少[85]。且另有研究指出,有氧运动干预后帕金森病患者血清中增加的BDNF水平与白细胞介素10/肿瘤坏死因子α的比值呈正相关,研究提示运动干预下BDNF所产生的神经保护作用会协同抗炎因子共同抵抗促炎症因子表达减轻帕金森病中的剧烈炎症反应[82]。
此外,帕金森病的病理状态下中枢神经炎症反应还可造成小胶质细胞细胞过度激活,进而使其正常的免疫监视和保护功能丧失转而产生更为严重的促炎负面效果,进一步加剧中枢神经炎症发生[93]。体外研究证实,BDNF预处理激活其下游信号通路表达后可促进细胞外信号调节激酶环磷酸腺苷反应元件结合蛋白信号通路表达,并抑制核转录因子κB上游调节因子糖原合成酶激酶3β表达,从而下调核转录因子κB转录水平以减少小胶质细胞活性[94]。动物模型研究表明,长期有氧运动促进帕金森病小鼠黑质中BDNF下游信号通路表达后可抑制小胶质细胞活化的标志物离子钙结合衔接分子1表达,进而减轻神经炎症反应[92]。然而,当特异性沉默原肌球蛋白相关激酶B表达以阻断BDNF下游信号通路激活后却发现上述效应消失,说明BDNF可通过介导运动抑制小胶质细胞过度激活进一步减轻神经炎症反应。
综上所述,直接证据表明BDNF可介导运动对小胶质细胞过度激活的抑制,间接证据发现BDNF还可通过协同抗炎因子共同抵抗促炎因子表达,从而减轻剧烈的神经炎症反应缓解帕金森病症状。
2.6.2 BDNF介导运动防止帕金森病中神经元异常凋亡 多巴胺能神经元异常凋亡是造成帕金森病疾病发生的主要病理机制之一,其诱发因素主要与多巴胺代谢紊乱以及α-突触核蛋白异常沉积等因素有关[2, 95]。有关帕金森病动物模型研究表明,有氧运动可通过上调BDNF-原肌球蛋白相关激酶B信号通路表达显著增加黑质和纹状体中酪氨酸羟化酶表达水平,进而促进多巴胺的合成释放以防止帕金森病小鼠中多巴胺能神经元损失[13, 49, 51, 53]。此外,区别于上述传统有氧运动,采用旋转杆步行训练的特殊有氧干预不仅通过上调腺苷酸活化蛋白激酶磷酸化水平诱导BDNF在多个脑区大量表达,还可在减少多巴胺能神经元损伤的同时保护其神经支配,促进黑质多巴胺能神经元向纹状体投射,以此进一步改善神经元分化缺陷[76],提示BDNF能够在脑中多个区域表达以提高神经元轴突传递活动,进而减少多巴胺能神经元损伤。对于帕金森病疾病所伴随的过度沉积的α-突触核蛋白而言,目前已有研究证实运动可作为减少α-突触核蛋白异位沉积的有效策略,2个月中等强度跑步机有氧运动(5-12 m/min,30 min,6 d/周)可显著抑制帕金森病小鼠脑内α-突触核蛋白预形成原纤维由黑质致密部向大脑皮质的扩散,进而激活脑内过氧化物酶体增殖物激活受体α表达保护黑质多巴胺能神经元免受神经毒素损伤[96]。LEEM等[77]的研究团队在新近研究中揭示了其中的内在机制,旋转杆步行训练可通过诱发帕金森病小鼠BDNF表达后抑制基质金属蛋白酶3和糖原合成酶激酶3β表达减少α-突触核蛋白在丝氨酸129位点的磷酸化修饰,继而降低α-突触核蛋白寡聚以减少路易小体的形成,从而减少多巴胺能神经元异常凋亡以促进神经元分化存活。
总之,BDNF可能通过介导运动维持多巴胺正常的代谢功能并降低α-突触核蛋白异位沉积,从而防止多巴胺能神经元异常凋亡并促进神经元分化与存活。
2.6.3 BDNF介导运动改善帕金森病中突触可塑性 帕金森病病理状态下神经元突触结构和功能被大量损坏,诱导长时程抑制效应发生,造成认知功能障碍发生。ANDRESKA等[97]研究表明,激活多巴胺第一受体后可诱发环磷酸腺苷磷酸化并促进原肌球蛋白相关激酶B向细胞表面易位,进而上调BDNF的敏感性,从而参与黑质中神经元突触可塑性的有效调控,但当突触前BDNF的缺失或突触后原肌球蛋白相关激酶B的抑制时均会抑制纹状体中棘神经元突触效能的长时程增强效应发生,提示BDNF改善神经元突触可塑性主要体现在可诱发突触效能的长时程增强效应发生,并且是其必不可少的关键诱发因子。学界对其背后可能的机制已系统阐明:当BDNF通过分泌囊泡运输至突触末端后,可由钙离子-L型电压门控通道介导的胞吐作用释放至细胞外,并在突触间隙中同时进行逆行及顺行信号传导[98]。在突触前膜上,BDNF与原肌球蛋白相关激酶B结合后提升胞浆内钙离子水平,促进谷氨酸的囊泡释放。而在突触后膜上,活化的原肌球蛋白相关激酶B进一步激活N-甲基-D-天冬氨酸及α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体,导致钙离子和钠离子在突触后膜的内流增加,从而引起微型兴奋性突触后电流的频率与幅度上升,最终促成突触效能的长时程增强效应发生。
值得注意的是,TANG等[55]在新近研究中发现BDNF还介导运动对突触结构和功能的重塑,8周有氧运动可激活帕金森病小鼠海马中BDNF下游信号通路进而促进突触后致密区蛋白95及突触素大量表达,继而增加突触活跃区长度以及突触后致密物质厚度,并显著提高树突及轴突数量以改善突触传递效率,从而达到增强神经元之间的连接和信息传递能改善突触可塑性。同时,上述突触改善效果在采用BDNF抑制剂(环曲霉素B)的运动组中则无法复现,说明BDNF相关信号通路的激活在运动改善突触可塑性中发挥关键作用。
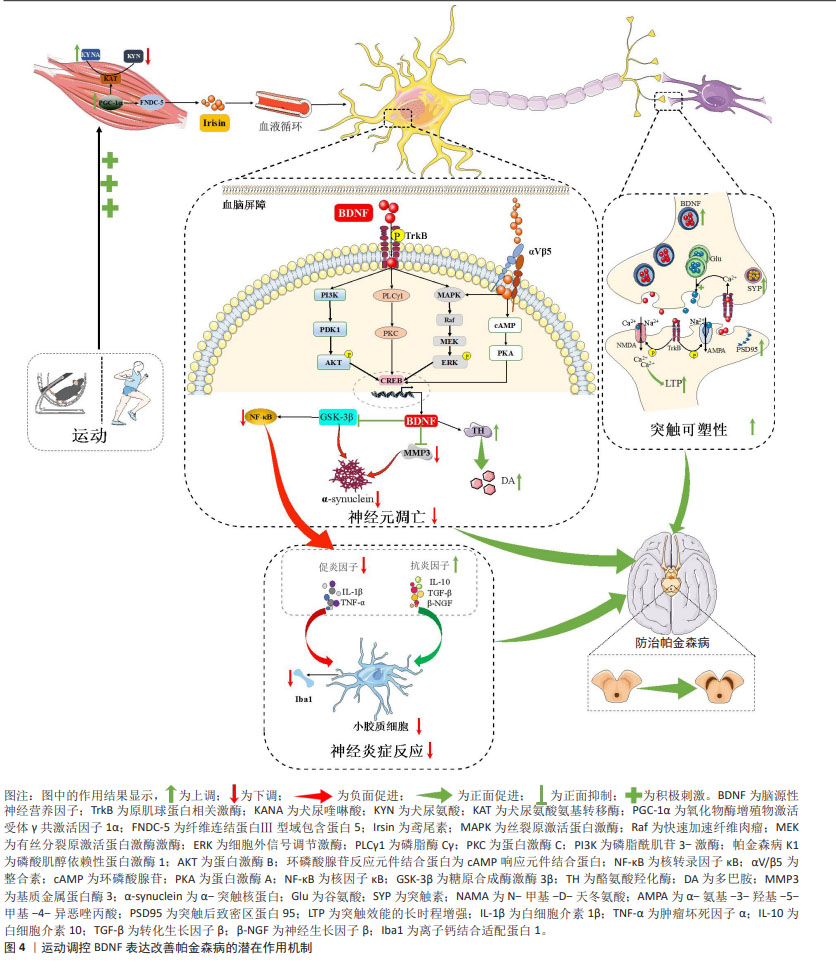
总之,BDNF可通过促进突触可塑性相关蛋白表达参与运动对突触结构和功能的调控,进而改善帕金森病中突触可塑性损伤。图4汇总了运动调控BDNF表达改善帕金森病的潜在作用机制。
| [1] BLOEM BR, OKUN MS, KLEIN C. Parkinson’s disease. The Lancet. 2021; 397(10291):2284-2303. [2] 黄镜璇,商慧芳.帕金森病的病因与发病机制研究进展[J].中国实用内科杂志,2023,43(10):797-801. [3] QI S, YIN P, WANG L, et al. Prevalence of Parkinson’s disease: a community-based study in China. Mov Disord. 2021;36(12):2940-2954. [4] OU R, HOU Y, WEI Q, et al. Longitudinal evolution of non-motor symptoms in early Parkinson’s disease: a 3-year prospective cohort study. NPJ Parkinsons Dis. 2021;7(1):58. [5] CENCI M A, RIGGARE S, PAHWA R, et al. Dyskinesia matters. Mov Disord. 2020;35(3):392-406. [6] 时凯旋,刘晓莉,乔德才.运动通过调节皮层-纹状体通路功能连接可塑性改善PD模型大鼠行为[J].体育科学,2020,40(6):10. [7] HARRO CC, SHOEMAKER MJ, COATNEY CM, et al. Effects of nordic walking exercise on gait, motor/non-motor symptoms, and serum brain-derived neurotrophic factor in individuals with Parkinson’s disease. Front Rehabil Sci. 2022;3:1010097. [8] CEFIS M, CHANEY R, WIRTZ J, et al. Molecular mechanisms underlying physical exercise-induced brain BDNF overproduction. Front Mol Neurosci. 2023;16:1275924. [9] PALASZ E, WYSOCKA A, GASIOROWSKA A, et al. BDNF as a promising therapeutic agent in Parkinson’s disease. Int J Mol Sci. 2020;21(3): 243-253. [10] MOGI M, TOGARI A, KONDO T, et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270(1):45-58. [11] HOWELLS DW, PORRITT MJ, WONG JY, et al. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol. 2000;166(1):127-135. [12] SCALZO P, KÜMMER A, BRETAS T L, et al. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol. 2010;257(4):540-555. [13] WU SY, WANG TF, YU L, et al. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25(1):135-146. [14] LI G, HUANG P, CUI S, et al. Tai Chi improves non-motor symptoms of Parkinson’s disease: One-year randomized controlled study with the investigation of mechanisms. Parkinsonism Relat Disord. 2024;120:105978. [15] BARDE YA, EDGAR D, THOENEN H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549-553. [16] AID T, KAZANTSEVA A, PIIRSOO M, et al. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85(3):525-535. [17] LUFT C, DA COSTA MS, ANTUNES GL, et al. The role of maternal exercise on placental, behavioral and genetic alterations induced by prenatal stress. Neurochem Int. 2022;158:105384. [18] INTLEKOFER KA, BERCHTOLD NC, MALVAEZ M, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38(10):2027-2034. [19] ARÉVALO JC, DEOGRACIAS R. Mechanisms controlling the expression and secretion of BDNF. Biomolecules. 2023. doi: 10.3390/biom13050789. [20] ALI NH, AL-KURAISHY HM, AL-GAREEB AI, et al. The molecular pathway of p75 neurotrophin receptor (p75NTR) in Parkinson’s disease: the way of new inroads. Mol Neurobiol. 2024;61(5):2469-2480. [21] HUANG EJ, REICHARDT LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609-642. [22] REICHARDT LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545-1564. [23] AHMED S, KWATRA M, GAWALI B, et al. Potential role of TrkB agonist in neuronal survival by promoting CREB/BDNF and PI3K/Akt signaling in vitro and in vivo model of 3-nitropropionic acid (3-NP)-induced neuronal death. Apoptosis. 2021;26(1-2):52-70. [24] LU B, PANG PT, WOO NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603-614. [25] LUO L, LI C, DU X, et al. Effect of aerobic exercise on BDNF/proBDNF expression in the ischemic hippocampus and depression recovery of rats after stroke. Behav Brain Res. 2019;362:323-331. [26] LUO L, LI C, DENG Y, et al. High-intensity interval training on neuroplasticity, balance between brain-derived neurotrophic factor and precursor brain-derived neurotrophic factor in poststroke depression rats. J Stroke Cerebrovasc Dis. 2019;28(3):672-682. [27] ZIEBELL M, KHALID U, KLEIN AB, et al. Striatal dopamine transporter binding correlates with serum BDNF levels in patients with striatal dopaminergic neurodegeneration. Neurobiol Aging. 2012;33(2):428-435. [28] PÅLHAGEN S, QI H, MÅRTENSSON B, et al. Monoamines, BDNF, IL-6 and corticosterone in CSF in patients with Parkinson’s disease and major depression. J Neurol. 2010;257(4):524-532. [29] LEVERENZ JB, WATSON GS, SHOFER J, et al. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(1):61-74. [30] MARTÍN DE PABLOS A, GARCÍA-MORENO JM, FERNÁNDEZ E. Does the cerebrospinal fluid reflect altered redox state but not neurotrophic support loss in Parkinson’s disease? Antioxid Redox Signal. 2015;23(11): 893-908. [31] AZEVEDO L, PEREIRA JR, SILVA SANTOS RM, et al. Acute exercise increases BDNF serum levels in patients with Parkinson’s disease regardless of depression or fatigue. Eur J Sport Sci. 2022;22(8):1296-1303. [32] YI X, YANG Y, ZHAO Z, et al. Serum mBDNF and ProBDNF expression levels as diagnosis clue for early stage Parkinson’s disease. Front Neurol. 2021;12:680765. [33] HUNG HC, LEE EH. The mesolimbic dopaminergic pathway is more resistant than the nigrostriatal dopaminergic pathway to MPTP and MPP+ toxicity: role of BDNF gene expression. Brain Res Mol Brain Res. 1996;41(1-2):14-26. [34] TSUKAHARA T, TAKEDA M, SHIMOHAMA S, et al. Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in monkeys. Neurosurgery. 1995;37(4):733-739; discussion 9-41. [35] KIM SR, KAREVA T, YARYGINA O, et al. AAV transduction of dopamine neurons with constitutively active Rheb protects from neurodegeneration and mediates axon regrowth. Mol Ther. 2012;20(2): 275-286. [36] NAM JH, LEEM E, JEON MT, et al. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: neuroprotective mechanisms of hRheb (S16H) in a model of Parkinson’s disease. Mol Neurobiol. 2015;51(2):487-499. [37] TRONCI E, NAPOLITANO F, MUÑOZ A, et al. BDNF over-expression induces striatal serotonin fiber sprouting and increases the susceptibility to l-DOPA-induced dyskinesia in 6-OHDA-lesioned rats. Exp Neurol. 2017;297:73-81. [38] HERNANDEZ-CHAN NG, BANNON MJ, OROZCO-BARRIOS CE, et al. Neurotensin-polyplex-mediated brain-derived neurotrophic factor gene delivery into nigral dopamine neurons prevents nigrostriatal degeneration in a rat model of early Parkinson’s disease. J Biomed Sci. 2015;22(1):59. [39] RAZGADO-HERNANDEZ LF, ESPADAS-ALVAREZ AJ, REYNA-VELAZQUEZ P, et al. The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS One. 2015;10(2):e0117391. [40] KHIDR HY, HASSAN NF, ABDELRAHMAN SS, et al. Formoterol attenuated mitochondrial dysfunction in rotenone-induced Parkinson’s disease in a rat model: role of PINK-1/PARKIN and PI3K/Akt/CREB/BDNF/TrKB axis. Int Immunopharmacol. 2023;125(Pt B):111207. [41] TENENBAUM L, HUMBERT-CLAUDE M. Glial cell line-derived neurotrophic factor gene delivery in Parkinson’s disease: a delicate balance between neuroprotection, trophic effects, and unwanted compensatory mechanisms. Front Neuroanat. 2017;11:29. [42] KHANAL S, BOK E, KIM J, et al. Dopaminergic neuroprotective effects of inosine in MPTP-induced parkinsonian mice via brain-derived neurotrophic factor upregulation. Neuropharmacology. 2023;238: 109652. [43] BUNKER DLJ. Delivery techniques in gene therapy: a brief overview. J Phys Chem Biophys. 2014;4(3):834-859. [44] WETTERGREN EE, QUINTINO L, MANFRÉ G, et al. Gene therapy for Parkinson’s disease. Drug Discov Today. 2002;7(2):88:664-677. [45] SAUCEDO MARQUEZ CM, VANAUDENAERDE B, TROOSTERS T, et al. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J Appl Physiol (1985). 2015;119(12):1363-1373. [46] CABRAL-SANTOS C, CASTRILLÓN CI, MIRANDA RA, et al. Corrigendum: inflammatory cytokines and BDNF response to high-intensity intermittent exercise: effect the exercise volume. Front Physiol. 2017; 7(12):437-449. [47] HWANG J, BROTHERS RM, CASTELLI DM, et al. Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neurosci Lett. 2016;630: 247-253. [48] O’CALLAGHAN A, HARVEY M, HOUGHTON D, et al. Comparing the influence of exercise intensity on brain-derived neurotrophic factor serum levels in people with Parkinson’s disease: a pilot study. Aging Clin Exp Res. 2020;32(9):1731-1758. [49] REAL CC, FERREIRA AF, CHAVES-KIRSTEN GP, et al. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson’s disease. Neuroscience. 2013;237:118-129. [50] TUON T, VALVASSORI SS, DAL PONT GC, et al. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res Bull. 2014;108:106-112. [51] DA COSTA RO, GADELHA-FILHO CVJ, DA COSTA A EM, et al. The treadmill exercise protects against dopaminergic neuron loss and brain oxidative stress in parkinsonian rats. Oxid Med Cell Longev. 2017;2017:2138169. [52] PALASZ E, NIEWIADOMSKI W, GASIOROWSKA A, et al. Neuroplasticity and neuroprotective effect of treadmill training in the chronic mouse model of Parkinson’s disease. Neural Plast. 2019;2019:8215017. [53] NIKOKALAM NAZIF N, KHOSRAVI M, AHMADI R, et al. Effect of treadmill exercise on catalepsy and the expression of the BDNF gene in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine -induced Parkinson in male NMRI mice. Iran J Basic Med Sci. 2020;23(4):483-493. [54] MARINO G, CAMPANELLI F, NATALE G, et al. Intensive exercise ameliorates motor and cognitive symptoms in experimental Parkinson’s disease restoring striatal synaptic plasticity. Sci Adv. 2023; 9(28):eadh1403. [55] TANG C, LIU M, ZHOU Z, et al. Treadmill exercise alleviates cognition disorder by activating the FNDC5:dual role of integrin αV/β5 in Parkinson’s disease. Int J Mol Sci. 2023;24(9):7830. [56] FRAZZITTA G, MAESTRI R, GHILARDI MF, et al. Intensive rehabilitation increases BDNF serum levels in parkinsonian patients: a randomized study. Neurorehabil Neural Repair. 2014;28(2):163-178. [57] ZOLADZ JA, MAJERCZAK J, ZELIGOWSKA E, et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J Physiol Pharmacol. 2014;65(3):441-458. [58] ANGELUCCI F, PIERMARIA J, GELFO F, et al. The effects of motor rehabilitation training on clinical symptoms and serum BDNF levels in Parkinson’s disease subjects. Can J Physiol Pharmacol. 2016;94(4):455-461. [59] ROTONDO R, PROIETTI S, PERLUIGI M, et al. Physical activity and neurotrophic factors as potential drivers of neuroplasticity in Parkinson’s disease: a systematic review and meta-analysis. Ageing Res Rev. 2023;92:102089. [60] EL HAYEK L, KHALIFEH M, ZIBARA V, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369-2382. [61] JIMÉNEZ-MALDONADO A, RENTERÍA I, GARCÍA-SUÁREZ PC, et al. The impact of high-intensity interval training on brain derived neurotrophic factor in brain: a mini-review. Front Neurosci. 2018;12:839. [62] 章森,邹勇,漆正堂,等.鸢尾素介导运动干预神经精神疾病的潜在机制[J].上海体育学院学报,2023,47(4):39-50. [63] 李刚强,耿小飞,周文辉,等.尿氨酸途径:帕金森病运动防治的一种可能新机制[J].中国体育科技,2023,59(9):63-71. [64] BOSTRÖM P, WU J, JEDRYCHOWSKI MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-478. [65] WRANN CD, WHITE JP, SALOGIANNNIS J, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649-659. [66] LOURENCO MV, FROZZA RL, DE FREITAS GB, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25(1):165-175. [67] LI D J, LI YH, YUAN HB, et al. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. 2017;68:31-42. [68] MARTIN KS, AZZOLINI M, LIRA RUAS J. The kynurenine connection:how exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am J Physiol Cell Physiol. 2020;318(5):C818-c830. [69] 徐琪坤,郝贵生.抑郁症与犬尿氨酸代谢及其产物关系的研究进展[J].神经疾病与精神卫生,2022,22(2):139-143. [70] PLATTEN M, NOLLEN E AA, RÖHRIG UF, et al. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18(5):379-401. [71] AGUDELO LZ, FEMENÍA T, ORHAN F, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159(1):33-45. [72] SODHI RK, BANSAL Y, SINGH R, et al. IDO-1 inhibition protects against neuroinflammation, oxidative stress and mitochondrial dysfunction in 6-OHDA induced murine model of Parkinson’s disease. Neurotoxicology. 2021;84:184-197. [73] IERACI A, BEGGIATO S, FERRARO L, et al. Kynurenine pathway is altered in BDNF Val66Met knock-in mice: effect of physical exercise. Brain Behav Immun. 2020;89:440-450. [74] IERACI A, MADAIO AI, MALLEI A, et al. Brain-derived neurotrophic factor Val66Met human polymorphism impairs the beneficial exercise-induced neurobiological changes in mice. Neuropsychopharmacology. 2016;41(13):3070-3089. [75] RAFIE F, RAJIZADEH MA, SHAHBAZI M, et al. Effects of voluntary, and forced exercises on neurotrophic factors and cognitive function in animal models of Parkinson’s disease. Neuropeptides. 2023;101:102357. [76] LEEM YH, PARK JS, PARK JE, et al. Neurogenic effects of rotarod walking exercise in subventricular zone, subgranular zone, and substantia nigra in MPTP-induced Parkinson’s disease mice. Sci Rep. 2022;12(1):10544. [77] LEEM YH, PARK JS, PARK JE, et al. Suppression of neuroinflammation and α-synuclein oligomerization by rotarod walking exercise in subacute MPTP model of Parkinson’s disease. Neurochem Int. 2023;165:105519. [78] SOKE F, KOCER B, FIDAN I, et al. Effects of task-oriented training combined with aerobic training on serum BDNF, GDNF, IGF-1, VEGF, TNF-α, and IL-1β levels in people with Parkinson’s disease: a randomized controlled study. Exp Gerontol. 2021;150:111384. [79] RAHMANI F, SAGHAZADEH A, RAHMANI M, et al. Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: a systematic review and meta-analysis. Brain Res. 2019;1704:127-136. [80] SEGURA C, ERASO M, BONILLA J, et al. Effect of a high-intensity tandem bicycle exercise program on clinical severity, functional magnetic resonance imaging, and plasma biomarkers in Parkinson’s disease. Front Neurol. 2020;11:656. [81] FERRUSOLA-PASTRANA A, DAVISON G, MEADOWS SN. The therapeutic effects of multimodal exercise for people with Parkinson’s: a longitudinal community-based study. Parkinsonism Relat Disord. 2023;110:105366. [82] LANDERS MR, NAVALTA JW, MURTISHAW AS, et al. A high-intensity exercise boot camp for persons with parkinson disease: a phase ii, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther. 2019;43(1):12-25. [83] STUCKENSCHNEIDER T, ABELN V, FOITSCHIK T, et al. Disease-inclusive exercise classes improve physical fitness and reduce depressive symptoms in individuals with and without Parkinson’s disease-A feasibility study. Brain Behav. 2021;11(10):e23-e52. [84] MACHADO S, TEIXEIRA D, MONTEIRO D, et al. Clinical applications of exercise in Parkinson’s disease:what we need to know? Expert Rev Neurother. 2022;22(9):771-780. [85] SZYMURA J, KUBICA J, WIECEK M, et al. The immunomodulary effects of systematic exercise in older adults and people with Parkinson’s disease. J Clin Med. 2020;9(1):184. [86] FREIDLE M, JOHANSSON H, EKMAN U, et al. Behavioural and neuroplastic effects of a double-blind randomised controlled balance exercise trial in people with Parkinson’s disease. NPJ Parkinsons Dis. 2022;8(1):12. [87] MUNHOZ RP, TUMAS V, PEDROSO JL, et al. The clinical diagnosis of Parkinson’s disease. Arq Neuropsiquiatr. 2024;82(6):1-10. [88] KWOK JYY, LEE JJ, CHOI E PH, et al. Stay mindfully active during the coronavirus pandemic: a feasibility study of mHealth-delivered mindfulness yoga program for people with Parkinson’s disease. BMC Complement Med Ther. 2022;22(1):37. [89] CHANDRA G, RANGASAMY SB, ROY A, et al. Neutralization of RANTES and eotaxin prevents the loss of dopaminergic neurons in a mouse model of Parkinson disease. J Biol Chem. 2016;291(29):15267-1581. [90] DA SILVA GERMANOS S, VIEIRA B, REICHERT VITAL DA SILVA I, et al. The impact of an aquatic exercise program on BDNF levels in Parkinson’s disease patients:short-and long-term outcomes. Funct Neurol. 2019; 34(2):65-70. [91] OLIVEIRA GS, IRACI L, PINHEIRO GS, et al. Effect of exercise and grape juice on epigenetic modulation and functional outcomes in PD: a randomized clinical trial. Physiol Behav. 2020;227:113135. [92] WANG TF, WU SY, PAN BS, et al. Inhibition of nigral microglial activation reduces age-related loss of dopaminergic neurons and motor deficits. Cells. 2022;11(3):481. [93] LULL ME, BLOCK ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7(4):354-365. [94] WU SY, PAN BS, TSAI SF, et al. BDNF reverses aging-related microglial activation. J Neuroinflammation. 2020;17(1):210. [95] 齐雪,李家慧,朱远峰,等.α-突触核蛋白的异常修饰及在帕金森病中的作用机制[J].中国组织工程研究,2024,28(8):1301-1306. [96] DUTTA D, PAIDI RK, RAHA S, et al. Treadmill exercise reduces α-synuclein spreading via PPARα. Cell Rep. 2022;40(2):111058. [97] ANDRESKA T, LÜNINGSCHRÖR P, WOLF D, et al. DRD1 signaling modulates TrkB turnover and BDNF sensitivity in direct pathway striatal medium spiny neurons. Cell Rep. 2023;42(6):112575. [98] CAMUSO S, LA ROSA P, FIORENZA MT, et al. Pleiotropic effects of BDNF on the cerebellum and hippocampus: implications for neurodevelopmental disorders. Neurobiol Dis. 2022;163:105606. |
| [1] | 董婷婷, 陈天鑫, 李 妍, 张 晟, 张 磊. 可干预因素与关节运动损伤的因果关系[J]. 中国组织工程研究, 2025, 29(9): 1953-1962. |
| [2] | 刘 琳, 刘世轩, 陆馨悦, 王 侃. 慢性肌筋膜触发点模型大鼠的尿液代谢组学分析[J]. 中国组织工程研究, 2025, 29(8): 1585-1592. |
| [3] | 王秋月, 靳 攀, 蒲 锐. 运动干预与细胞焦亡在骨关节炎中的作用[J]. 中国组织工程研究, 2025, 29(8): 1667-1675. |
| [4] | 张孜贤, 徐有粮, 吴绍奎, 王相英. 血流限制训练法联合抗阻训练对运动者肌肉相关指标影响的Meta分析[J]. 中国组织工程研究, 2025, 29(8): 1705-1713. |
| [5] | 王 娟, 王广兰, 左会武. 运动疗法对前交叉韧带重建后康复疗效影响的网状Meta分析[J]. 中国组织工程研究, 2025, 29(8): 1714-1726. |
| [6] | 郑华坤, 殷明越, 刘 骞. 间歇与持续训练对体力活动不足成人生活质量影响的Meta分析[J]. 中国组织工程研究, 2025, 29(8): 1727-1740. |
| [7] | 娄 国, 张 敏, 付常喜. 8周运动预适应增强脂肪干细胞治疗心肌梗死大鼠的效果[J]. 中国组织工程研究, 2025, 29(7): 1363-1370. |
| [8] | 郑荣发, 莫伟彬, 黄 鹏, 陈俊吉, 梁 婷, 资方宇, 李国峰. 电针对运动大鼠腓肠肌组织代谢酶及自噬基因表达的影响[J]. 中国组织工程研究, 2025, 29(6): 1127-1136. |
| [9] | 刘哲哲, 于梅青, 王婷婷, 张 敏, 李百艳. 曲克芦丁调控核因子κB信号通路抑制脑梗死模型大鼠脑损伤及神经元凋亡[J]. 中国组织工程研究, 2025, 29(6): 1137-1143. |
| [10] | 陈玉宁, 蒋 颖, 廖翔宇, 陈琼君, 熊 亮, 刘 悦, 刘 通. 补气活血合剂干预脑缺血再灌注模型大鼠相关因子及自噬蛋白的表达[J]. 中国组织工程研究, 2025, 29(6): 1152-1158. |
| [11] | 赵晓璇, 刘帅祎, 李 奇, 邢 政, 李庆雯, 褚晓蕾. 不同运动方式促进周围神经损伤后的功能恢复[J]. 中国组织工程研究, 2025, 29(6): 1248-1256. |
| [12] | 张文华, 李 荀, 张伟超, 李欣颖, 马帼澳, 王孝强. SphK1/S1P/S1PR2信号通路促进肌生成:运动改善骨骼肌健康的新视角[J]. 中国组织工程研究, 2025, 29(6): 1265-1275. |
| [13] | 马浩宇, 乔鸿超, 郝茜茜, 史冬博. 不同运动强度与骨关节炎发病风险的效应分析[J]. 中国组织工程研究, 2025, 29(6): 1305-1311. |
| [14] | 逯冉冉, 周 旭, 张利杰, 杨新玲. 富马酸二甲酯减轻帕金森病模型鼠神经损伤的作用机制[J]. 中国组织工程研究, 2025, 29(5): 989-994. |
| [15] | 王东阳, 杨巧慧, 林欣潮. 绝经后女性维生素D水平与生殖特点和运动膳食情况的关系[J]. 中国组织工程研究, 2025, 29(5): 1021-1025. |
运动对中枢系统的保护作用主要得益于能够诱导大量神经保护因子表达,并通过加强不同器官及组织之间的交互作用,进而延缓帕金森病发生发展。脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)是运动诱导产生的主要神经保护因子之一,其在中枢神经系统中调控神经系统稳态、促进神经元存活及突触可塑性、加强学习和记忆等方面发挥关键作用,因此被视为内源性“神经保护剂”[8]。研究表明,帕金森病疾病风险增加及发生发展与神经炎症反应加剧、多巴胺耗竭、α-突触核蛋白和神经元异常凋亡等方面有关,而其中所涉及的免疫系统、单胺能系统以及神经中枢稳态系统的共同调节因子是BDNF[9],提示BDNF在运动改善帕金森病中扮演着重要角色。尽管目前已有学者综述了BDNF在运动改善帕金森病过程中的相关作用机制,但更多是基于多种神经退行性疾病(同时包括帕金森病和阿尔茨海默病)共同探讨的结果[9],这可能无法充分反映帕金森病的特异性病理特征,缺乏一定指向性。考虑到帕金森病在不同阶段的临床表现具有显著差异,帕金森病动物模型和患者机体内的BDNF表达及对运动干预的响应也可能存在一定差异,因此目前关于BDNF介导下运动改善帕金森病的机制研究缺乏对帕金森病特有病理的深入探讨。同时,不同类型的运动干预对BDNF的调控作用以及其在帕金森病治疗中的效果差异,尚未进行详尽的比较分析。这些差异对于制定个性化的帕金森病运动干预策略至关重要,当前研究的局限性可能制约了针对帕金森病精准运动治疗方案的设计和发展。此外,绝大多数研究均围绕运动对帕金森病运动症状的改善情况,而忽视帕金森病发展过程中的非运动症状如睡眠障碍的变化,进一步制约研究结果的普适性。
基于此,文章就BDNF的表达及分泌、BDNF和帕金森病的关系为切入点进行文献综述,着重分析帕金森病病理状态下运动对BDNF表达的特异性调控,并聚焦于BDNF介导下不同运动方式对帕金森病改善效果及潜在作用机制的最新进展,以期为帕金森病临床干预和运动康复提供新思路和理论基础。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者于2023年6月至2024年2月30日通过数据库进行检索。
1.1.2 检索文献时限 2000-01-01/2024-02-30,重点检索2018年1月至2023年10月发表的文献,同时纳入少数早期经典及特别相关文献。
1.1.3 检索数据库 中国知网、万方数据库、PubMed和Web of Science数据库。
1.1.4 检索途径 主题词、关键词、摘要和全文检索。
1.1.5 检索词 中文检索词为“帕金森病,脑源性神经营养因子,神经保护,多巴胺,神经元异常凋亡,神经炎症反应,突触可塑性,运动”;英文检索词为“Parkinson’s disease,BDNF,Neuroprotection,synaptic plasticity”。
1.1.6 检索文献类型 综述、荟萃分析、述评、临床试验研究和期刊论文。
1.1.7 手工检索情况 对文献中高价值的参考文献首先阅读文题,再以标题进行检索,阅读摘要或全文。
1.1.8 文献检索策略 以PubMed和万方数据库检索策略为例,见图1。
1.1.9 检索文献量 初检文献584篇,其中中文文献238篇,英文文献346篇;中国知网数据库143篇,万方数据库95篇,PubMed数据库194篇,Web of Science数据库152篇。
1.2 入组标准1.2.1 纳入标准 ①该综述所纳入的实验性文献类型所采用的实验设计应为随机对照的方式,研究对象与方法必须有明确告知实验组(试验组)以及对照组,且实验中各分组的样本在年龄和性别等方面呈均匀分配;②研究对象必须为帕金森病患者或帕金森病动物模型;③实验设计必须围绕运动进行。
1.2.2 排除标准 ①文献中没有详细的实验流程、实验数据及实验结果分析;②研究对象不明确,帕金森病患者的疾病情况未告知,帕金森病动物模型中的造模方式未告知;③研究设计中的运动处方未告知。
1.3 文献质量评估 严格按照上述纳排标准进行文献筛选,文献质量信度及效度的评判方式是采用皮卓量表(简称PEDro)予以评分,在每项评分标准下评判文献的研究品质,判断其是否符合文章的纳入标准。研究最终纳入高质量文献98篇进行综述,包括中国知网5篇、万方数据库1篇、PubMed数据库64篇及Web of Science数据库28篇文献。文献筛选流程图见图2。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
3.2 综述主要的特色与价值 该综述通过对近10年BDNF介导下运动防治帕金森病的相关研究进行全面归纳、比较和评述,尤其区别与以往研究更加具体深刻地探讨了帕金森病病理背景下运动对BDNF表达的调控作用和机制,并详细梳理不同运动类型在BDNF介导下运动对帕金森病的改善效果,与此同时拓展运动干预视角纳入最新身心运动的相关研究,并强调了身心运动在帕金森病发展过程中非运动症状治疗中的潜在神经保护作用,最后在以往研究基础上进一步完善BDNF介导下运动改善帕金森病的潜在作用机制。此综述结果显示,①在帕金森病病理背景下,运动可通过促进肌因子鸢尾素大量分泌,并降低色氨酸-犬尿氨酸代谢途径紊乱特异性调控BDNF表达。②有氧运动,尤其是特殊有氧运动(动物:旋转杆步行/人类:北欧健走)以及多模式运动可显著上调脑源性神经营养因子表达,进而改善帕金森病的运动症状,此外脑源性神经营养因子还介导身心运动(太极拳)对帕金森病患者认知障碍和睡眠障碍等非运动症状的有效调节。③运动诱导的高表达脑源性神经营养因子可能通过:上调抗炎因子白细胞介素10、神经生长因子β和转化生长因子β表达、下调促炎因子肿瘤坏死因子α及白细胞介素1β表达,并抑制核转录因子κB信号通路表达降低小胶质细胞活性减轻神经炎症反应;增加酪氨酸羟化酶活性以促进多巴胺合成释放,并通过下调基质金属蛋白酶3及糖原合成酶激酶3β表达抑制α-突触核蛋白在丝氨酸129位点的磷酸化修饰,以防止神经异常凋亡;诱导突触效能的长时程增强效应发生、促进突触后致密区蛋白95及突触素大量表达改善突触可塑性发挥神经保护作用延缓帕金森病发生发展。 3.3 综述的局限性 该综述主要聚焦于BDNF介导下不同运动类型对帕金森病的改善效果,而对运动处方的其他要素,如强度、周期及频率等对治疗效果的影响分析不足。就运动强度而言,LANDERS等[82]发现在8周多模式运动干预下高强度运动似乎比低强度运动带来更好的帕金森病改善效果,同时帕金森病患者对高强度多模式运动的依从性、不良事件发生率均与低强度运动无显著差异,在运动频率方面,3次/周的多模式运动可显著提高帕金森病患者的BDNF表达水平并改善其运动症状,但2周/次的多模式运动干预无法提高帕金森病患者的认知功能且血清BDNF水平也无显著影响[83],然而鉴于目前研究选择偏差、数据异质性、统计分析方法的复杂性,以及BDNF测量技术的差异,加之帕金森病病理机制的多维性和现有研究中长期随访数据的匮乏,进一步阻碍了对BDNF与帕金森病病理之间动态关系的深入理解。因此,对于处于不同病理时期的帕金森病患者而言,采用何种类型、强度、周期和频率的运动处方能够诱导BDNF发挥最佳神经肌肉保护效应暂无定论,未来有必要进行详细的归纳,分析和探讨。
3.4 综述的重要意义 该综述通过对帕金森病病理背景下运动调控BDNF表达的作用和机制进行详细分析,梳理不同类型的运动对帕金森病的改善效果,聚焦于BDNF介导下运动防治帕金森病潜在作用机制,将有助于推动靶向BDNF的帕金森病运动治疗策略朝向“运动+药物”精准医疗的发展,为帕金森病临床干预和运动康复提供新思路和理论基础。
3.5 建议与展望 鉴于帕金森病动物模型及人体临床研究所显示不同运动处方下BDNF调控作用及帕金森病改善效果的诸多矛盾,围绕BDNF进行运动改善帕金森病的更深入靶向研究在于:①在干预指标选取方面:目前研究普遍集中探讨BDNF表达的变化情况,鲜有研究纳入proBDNF指标的变化情况,而后者可激活c-Jun N-末端激酶信号通路表达启动凋亡联级反应进而导致神经细胞凋亡,加剧帕金森病发生。因此运动是否能够发挥双向调控的作用:在上调BDNF表达的同时还协同抑制proBDNF表达从而减轻BDNF/proBDNF失衡有待进一步验证。②在干预效果方面:尽管有氧运动,多模式运动和平衡运动可显著调控BDNF表达,有效改善帕金森病运动症状。但目前研究缺乏对采用单独抗阻运动干预模式的探索;此外,由于帕金森病疾病的复杂性帕金森病患者在不同疾病阶段运动症状有所差异,不同时期机体内BDNF表达水平以及对运动的响应程度可能存在一定差异,因此亟待学者采用更加统一和系统的运动处方,围绕非有氧运动类型,如抗阻和身心运动,并对同一批帕金森病患者进行长期纵向跟踪研究,以此完善帕金森病运动防治领域研究的不足。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
帕金森病:是一种神经退行性疾病,主要发生于中老年人。帕金森病的主要病理特征为中脑黑质致密部多巴胺能神经元异常凋亡,以及错误折叠的α-突触核蛋白异常沉积,产生嗜酸性神经元包涵体——路易小体,同时还伴随剧烈的神经免疫炎症反应。#br# 脑源性神经影响因子:是继神经生长因子之后,在猪脑中纯化和表征神经源性蛋白后发现的第二个神经营养因子,其在中枢神经系统中调控神经系统稳态、促进神经元存活及突触可塑性、加强学习和记忆等方面发挥关键作用,因此被视为内源性“神经保护剂”。#br##br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
运动干预作为经济有效的非物理疗法,可有效上调脑源性神经营养因子表达进而防治帕金森病发生发展,但目前关于靶向脑源性神经营养因子的运动治疗策略在延缓帕金森病发生发展的潜在作用机制尚不明晰。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||