中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (25): 5443-5453.doi: 10.12307/2025.515
• 干细胞综述 stem cell review • 上一篇 下一篇
细胞之间线粒体转移的主要途径、主要作用及主要调控机制
李兴福1,2,周光前1,陆 伟1,2
- 1深圳大学医学部生物医学工程学院,医学超声关键技术国家地方联合工程实验室,广东省生物医学信息检测与超声成像重点实验室,广东省深圳市 518060;2深圳市第二人民医院运动医学科,广东省深圳市 518035
-
收稿日期:2024-05-20接受日期:2024-06-12出版日期:2025-09-08发布日期:2024-12-28 -
通讯作者:陆伟,主任医师,博士,深圳大学医学部生物医学工程学院,医学超声关键技术国家地方联合工程实验室,广东省生物医学信息检测与超声成像重点实验室,广东省深圳市 518060;深圳市第二人民医院运动医学科,广东省深圳市 518035 -
作者简介:李兴福,男,1988年生,山东省济宁市人,2021年广州医科大学毕业,博士,医师,主要从事运动医学及骨组织工程研究。 并列第一作者:周光前,男,1962年生,贵州省遵义市人,2000年瑞典宇默尔大学毕业,博士,教授,主要从事干细胞工程研究。 -
基金资助:国家自然科学基金面上项目(82072515),项目负责人:陆伟;深圳市科创委基础研究面上项目(JCYJ20220530150615035),项目负责人:李兴福
Main pathways, roles, and regulatory mechanisms of intercellular mitochondrial transfer
Li Xingfu1, 2, Zhou Guangqian1, Lu Wei1, 2
- 1Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, School of Biomedical Engineering, Shenzhen University Medical School, Shenzhen 518060, Guangdong Province, China; 2Department of Sports Medicine, Shenzhen Second People’s Hospital, Shenzhen 518035, Guangdong Province, China
-
Received:2024-05-20Accepted:2024-06-12Online:2025-09-08Published:2024-12-28 -
Contact:Lu Wei, PhD, Chief physician, Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, School of Biomedical Engineering, Shenzhen University Medical School, Shenzhen 518060, Guangdong Province, China; Department of Sports Medicine, Shenzhen Second People’s Hospital, Shenzhen 518035, Guangdong Province, China -
About author:Li Xingfu, MD, Physician, Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, School of Biomedical Engineering, Shenzhen University Medical School, Shenzhen 518060, Guangdong Province, China; Department of Sports Medicine, Shenzhen Second People’s Hospital, Shenzhen 518035, Guangdong Province, China; Zhou Guangqian, PhD, Professor, Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, School of Biomedical Engineering, Shenzhen University Medical School, Shenzhen 518060, Guangdong Province, China -
Supported by:National Natural Science Foundation of China, No. 82072515 (to LW); Fundamental Research Program of Shenzhen Science and Technology Innovation Commission, No. JCYJ20220530150615035 (to LXF)
摘要:
文题释义:
线粒体:是一种由两层膜包被的半自主细胞器,广泛存在于真核细胞,具有合成能量的功能,是胞内有氧呼吸的主要场所,在细胞生物学行为中发挥重要作用,被称为“细胞动力站”,其直径为0.5-1.0 µm。线粒体转移:指线粒体通过各种途径进入相邻细胞,或者脱离母细胞形成游离线粒体并前往远处宿主细胞的过程。
摘要
背景:线粒体功能障碍导致细胞衰老凋亡,可加重组织损伤。细胞之间线粒体转移促进损伤细胞恢复线粒体功能,有助于治疗线粒体相关疾病。
目的:综述细胞之间线粒体转移的作用及调控机制。
方法:检索中国知网和PubMed数据库2014-2024年关于细胞之间线粒体转移的文献,以“线粒体转移,隧道纳米管,缝隙连接,微囊泡,细胞融合”为中文检索词,以“Mitochondrial transfer,Tunneling nanotubes,gap junctions,microvesicles,cell fusion”为英文检索词,最终共纳入74篇文献进行分析。
结果与结论:①总结了细胞之间线粒体转移的4个主要途径:包括隧道纳米管、缝隙连接、细胞融合和微囊泡。②梳理了细胞之间线粒体转移的主要作用,包括物质交换、信息传递、改善宿主细胞线粒体功能、抑制氧化应激、提高细胞增殖活力及抗炎抗衰老等。③总结了细胞之间线粒体转移的主要调控机制,包括Miro 1促进隧道纳米管形成和线粒体转移、隧道纳米管转移线粒体依赖宿主细胞环状ADP核糖水解酶表达、氧化应激环境诱导隧道纳米管形成、缝隙连接具有Ca2+依赖性、缝隙连接蛋白43影响缝隙连接形成、激活Ras1蛋白和肌动蛋白有助于细胞融合、肌动蛋白和Rab6参与调控线粒体出胞、激活肌动蛋白和NAD+-CD38-cADPR-Ca2+信号通路促进线粒体入胞等。④在细胞信号传导蛋白、细胞动力学相关蛋白及氧化应激环境的影响下,细胞通过隧道纳米管、缝隙连接、微囊泡及细胞融合进行线粒体转移。⑤线粒体转移是细胞之间物质交换和信息交流的重要途径,与疾病的发生发展息息相关,可为治疗线粒体相关疾病提供新的思路,但对细胞间线粒体转移的作用及调控机制仍需进一步探究。
中图分类号:
引用本文
李兴福, 周光前, 陆 伟. 细胞之间线粒体转移的主要途径、主要作用及主要调控机制[J]. 中国组织工程研究, 2025, 29(25): 5443-5453.
Li Xingfu, Zhou Guangqian, Lu Wei. Main pathways, roles, and regulatory mechanisms of intercellular mitochondrial transfer[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5443-5453.
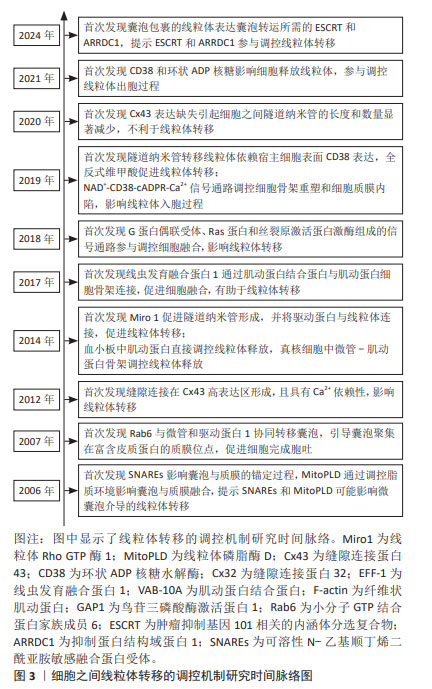
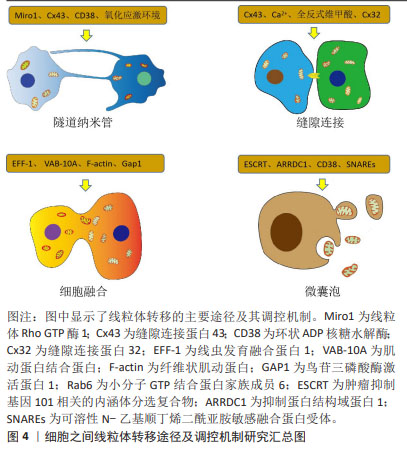
2.1 细胞接触状态下线粒体转移的作用及分子调控机制 在体内外环境中,细胞之间存在线粒体转移现象,线粒体转移是细胞之间物质交换和信息交流的重要方式。在接触状态下细胞可通过隧道纳米管、缝隙连接及细胞融合完成线粒体转移。供体细胞线粒体转移可改善宿主细胞线粒体功能,减轻氧化应激损伤,挽救宿主细胞命运。
2.1.1 细胞通过隧道纳米管转移线粒体的作用及分子调控机制 隧道纳米管是基于细胞骨架形成的细胞之间管道样结构,主要介导细胞间物质交换,包括脂质、核酸、蛋白质甚至细胞器等,在人体病理生理过程中发挥重要作用[13]。许多研究已经证实,隧道纳米管是细胞之间线粒体转移的重要途径,在线粒体供体细胞调控宿主细胞生物学行为过程中发挥巨大作用,而隧道纳米管的形成受到氧化应激环境、线粒体Rho GTP酶、缝隙连接蛋白和环状ADP核糖水解酶等因素的影响。
在干细胞研究领域,AHMAD等[9]发现多能间充质干细胞通过隧道纳米管将线粒体转移到神经细胞,线粒体Rho GTP酶1可促进隧道纳米管形成,并将驱动蛋白家族成员5(kinesin family member 5,KIF 5)与线粒体连接,促进多能间充质干细胞线粒体转移到神经细胞。ZHANG等[14]发现间充质干细胞通过隧道纳米管将线粒体转移到急性肺损伤模型的肺血管内皮细胞,可有效改善肺血管的通透性并减轻肺水肿。LIN等[15]发现间充质干细胞通过隧道纳米管将线粒体转移到氧化应激损伤的血管内皮细胞,而阻断隧道纳米管形成会损害血管内皮细胞,并且发现人工移植间充质干细胞线粒体到血管内皮细胞可短暂地增强能量合成并促进功能性血管生成,但人工移植线粒体没有整合到内皮细胞线粒体网络,而是在内化后引发了线粒体自噬。JIANG等[16]发现间充质干细胞通过隧道纳米管将线粒体转移到各种眼部细胞(包括角膜内皮、视网膜色素上皮和光感受器细胞),可有效减轻眼部细胞的氧化应激损伤,并发现线粒体转移效率与隧道纳米管数量呈正相关,而鱼藤酮诱导的氧化应激可提高线粒体的转移效率。SAGAR等[17]发现间充质干细胞通过隧道纳米管转移线粒体到小鼠呼吸道上皮细胞,可改善上皮细胞线粒体功能并减轻哮喘炎症反应,同时降低Th细胞因子分泌水平。目前国内研究也支持间充质干细胞通过线粒体转移保护损伤的宿主细胞,而隧道纳米管是间充质干细胞线粒体转移的重要途径[18]。以上研究说明,间充质干细胞通过隧道纳米管将自身线粒体转移到损伤宿主细胞,可改善宿主细胞线粒体功能,降低氧化应激水平,进而减轻组织损伤。
在肿瘤细胞研究领域,GOLIWAS等[7]发现癌症相关成纤维细胞通过隧道纳米管将线粒体转移转移到乳腺癌细胞并增强其迁移能力。AMIN等[19]发现大鼠嗜铬细胞瘤PC12细胞之间存在隧道纳米管,可作为物质交换通道参与质膜囊泡和细胞器的选择性转移;而WANG等[20]发现嗜铬细胞瘤PC12细胞可通过隧道纳米管将线粒体转移到应激损伤细胞并逆转其早期凋亡状态,还发现隧道纳米管是由应激细胞产生,且线粒体转移方向是从健康细胞到应激损伤细胞。WANG等[21]发现白血病细胞通过隧道纳米管将自身线粒体转移到间充质干细胞,进而避免氧化应激损伤,但间充质干细胞的氧化应激水平显著升高。TISHCHENKO等[22]发现缝隙连接蛋白43表达缺失引起乳腺癌细胞系中隧道纳米管长度和数量的显著减少,提示缝隙连接蛋白43在隧道纳米管形成过程中起到重要调控作用。以上研究说明线粒体转移同样可以挽救肿瘤细胞命运,从而对人体造成不良影响。因此,在调控线粒体转移治疗临床疾病时要根据具体情况采用针对性策略。
在心血管细胞研究领域,KOYANAGI等[23]发现心肌细胞通过隧道纳米管将功能障碍线粒体转移到内皮祖细胞的现象,避免心肌细胞受到氧化应激损伤,由此可见线粒体转移也是损伤细胞自我保护的重要机制。在基质细胞研究领域,SAITO等[24]发现骨髓基质细胞通过隧道纳米管将线粒体转移到急性髓性白血病细胞,有效增强了急性髓性白血病细胞的能量代谢水平。BURT等[25]发现间充质基质细胞系HS27a细胞通过隧道纳米管将线粒体转移到急性淋巴细胞白血病细胞,可减轻急性淋巴细胞白血病细胞应激损伤。BOISE等[26]发现骨髓基质细胞通过隧道纳米管将线粒体转移到骨髓瘤细胞并促进其增殖,而隧道纳米管转移线粒体依赖骨髓瘤细胞表面环状ADP核糖水解酶的表达,NI等[27]的研究结果也印证了这一观点。以上研究说明,各类基质细胞可以通过隧道纳米管转移线粒体到肿瘤细胞,导致肿瘤细胞活力增强。针对基质细胞线粒体转移对肿瘤细胞的保护作用,阻断隧道纳米管可能有助于肿瘤疾病的治疗。
总之,隧道纳米管是细胞之间线粒体转移的重要途径,可有效降低宿主细胞的氧化应激水平,改善线粒体功能,挽救细胞命运。损伤细胞也可以通过隧道纳米管转移功能障碍的线粒体进行自我保护。线粒体转移对机体生理功能的影响具有双向性,正常线粒体转移到正常组织的受损细胞可有效改善其线粒体功能并减轻组织损伤,而正常线粒体转移到肿瘤组织可增强肿瘤细胞的抗药性和迁移能力。因此,在调控细胞之间线粒体转移治疗疾病时要根据组织情况决定调控策略。隧道纳米管的形成受到氧化应激环境、线粒体Rho GTP酶1、缝隙连接蛋白43及环状ADP核糖水解酶等因素的影响,所以调控细胞之间线粒体转移可将以上因素作为调控靶点。
2.1.2 细胞通过缝隙连接转移线粒体的作用及分子调控机制 缝隙连接是由连接子蛋白复合物形成的细胞膜阵列嵌入通道,具有传递细胞信号的功能,参与多种离子、蛋白分子及细胞器的转运[28]。许多研究已经证实,细胞相互接触时通过缝隙连接转移线粒体能够改变宿主细胞的生物学行为,在调控机体病理生理过程中发挥巨大作用,而缝隙连接的形成受到缝隙连接蛋白、钙离子及缝隙连接增效/抑制剂等因素的影响。
在肿瘤细胞研究领域,ZAMPIERI等[29]认为缝隙连接参与肿瘤细胞和正常细胞之间的线粒体转移,正常细胞通过缝隙连接转移线粒体影响肿瘤细胞迁移、侵袭和治疗抵抗等生物学行为。SINGH等[30]发现缝隙连接参与白血病细胞和正常细胞之间的线粒体转移,影响骨髓抑制后的再生造血;转移的线粒体可提高造血细胞能量合成,增强造血细胞活力,有助于改善肿瘤化疗预后。以上研究说明,缝隙连接参与下的线粒体转移在肿瘤发展过程中针对不同肿瘤类型发挥不同作用,需要辨证地看待线粒体转移对机体病理生理的影响。
在干细胞研究领域,ISLAM等[10]发现骨髓间充质干细胞通过缝隙连接将线粒体转移到肺泡上皮细胞,可有效降低上皮细胞的氧化应激水平,抑制炎性反应;骨髓间充质干细胞更易黏附在肺泡上皮的Cx43高表达区,提示缝隙连接蛋白43可能影响缝隙连接形成;负载钙离子螯合剂的骨髓间充质干细胞更易附着在肺泡上并与肺泡上皮细胞形成缝隙连接,提示缝隙连接具有钙离子依赖性。LI等[31]
研究发现骨髓间充质干细胞通过缝隙连接将自身线粒体转移到损伤的运动神经元细胞,可有效改善能量供给,减少神经元细胞凋亡;全反式维甲酸(缝隙连接增效剂)促进线粒体从骨髓间充质干细胞转移到神经元,而18β-甘草次酸(缝隙连接抑制剂)抑制线粒体转移;骨髓间充质干细胞表达缝隙连接蛋白43,而运动神经元细胞表达缝隙连接蛋白32,提示骨髓间充质干细胞可能依靠缝隙连接蛋白43和缝隙连接蛋白32形成的异型缝隙连接转移线粒体到运动神经元细胞。ANDREW等[32]发现骨髓间充质干细胞依靠非缝隙连接蛋白43向支气管上皮细胞转移线粒体,这与ISLAM等[10]发现的骨髓间充质干细胞依靠缝隙连接蛋白43向肺泡上皮细胞转移线粒体的现象相悖;支气管上皮细胞不能向骨髓间充质干细胞转移线粒体,提示骨髓间充质干细胞通过缝隙连接转移线粒体具有单向性。国内学者认为缝隙连接蛋白43通过直接构成缝隙连接参与人体病理生理过程,应该特别关注缝隙连接蛋白43结构功能的完整性[33]。另外,有研究表明体内环境下细胞通过缝隙连接转移线粒体有助于维持机体内环境稳定[34-35]。以上研究说明,干细胞通过缝隙连接将线粒体转移到受损宿主细胞可有效改善宿主细胞的线粒体功能,进而减轻组织损伤。因此,在干细胞治疗过程中,促进缝隙连接形成或许可以提高干细胞的治疗效果。
总之,缝隙连接是细胞之间线粒体转移的重要途径,可有效改善宿主细胞线粒体功能,影响宿主细胞的命运。缝隙连接参与的线粒体转移对肿瘤病理发展的影响具有两面性,在调控线粒体转移治疗肿瘤时要根据组织类型决定调控策略。干细胞通过缝隙连接转移线粒体修复组织损伤是干细胞发挥保护作用的重要途径,促进干细胞与损伤细胞之间形成缝隙连接可能提高干细胞治疗效率。缝隙连接的形成受到缝隙连接蛋白43、缝隙连接蛋白32、钙离子、全反式维甲酸和18β-甘草次酸等因素的影响,研究者们或许可将以上因素作为调控线粒体转移的靶点,应用于临床疾病治疗。
2.1.3 细胞通过细胞融合转移线粒体的作用及分子调控机制 细胞融合是两个独立细胞发生细胞膜融合,共享细胞器和胞内化合物,而细胞核均保持独立完整的过程。细胞部分融合时可发生短暂直接的细胞间通讯和多种细胞器交换,包括线粒体转移[36]。虽然有研究发现细胞融合过程中存在线粒体转移现象,但是在细胞融合状态下难以准确检测线粒体转移的作用。另外,许多研究者普遍认为细胞融合过程主要受到细胞动力学相关蛋白的调控。
在胚胎细胞研究领域,YANG等[11]研究发现线虫发育融合蛋白1通过肌动蛋白结合蛋白(spectraplakin/VAB-10A)与肌动蛋白细胞骨架连接,促进胚胎细胞融合;与肌动蛋白结合蛋白交联的肌动蛋白可以招募融合素到细胞融合位点,有助于细胞融合;线虫发育融合蛋白1在体外环境下可促进肌动蛋白结合蛋白与纤维状肌动蛋白结合,而在线虫发育融合蛋白1或肌动蛋白结合蛋白突变体中,纤维状肌动蛋白动力学性能降低,不利于细胞融合。WHITLOCK等[37]发现降低磷脂酰丝氨酸和磷脂酰丝氨酸识别蛋白的表达水平可抑制胚胎细胞融合过程。以上研究说明,细胞动力学相关蛋白参与下的细胞融合在机体生长发育过程中发挥重要作用。
在成体细胞研究领域中,SMUROVA等[38]发现表皮细胞顶膜区域富集EFF-1的位置可发生质膜融合,线虫发育融合蛋白1可被动力蛋白和小GTP结合蛋白家族成员5依赖的内吞作用主动清除,而下调动力蛋白和小GTP结合蛋白家族成员5表达水平会导致细胞过度融合。MERLINI等[39]发现由G蛋白偶联受体、大鼠肉瘤蛋白和丝裂原激活蛋白激酶组成的信号通路参与调控细胞融合过程;激活大鼠肉瘤蛋白1可促进细胞融合,而大鼠肉瘤蛋白1活性减弱可抑制细胞融合过程;鸟苷三磷酸酶激活蛋白1可抑制大鼠肉瘤蛋白1的活性,募集到大鼠肉瘤蛋白1位点的鸟苷三磷酸酶激活蛋白1可抑制细胞融合。DUDIN等[40]发现真核细胞的融合过程由G蛋白偶联受体-丝裂原活化蛋白激酶信号级联的富集触发,而自分泌细胞可独立开启细胞融合;在细胞融合过程中,细胞释放信息素聚集在肌动蛋白融合焦点,而信息素受体和丝裂原活化蛋白激酶信号级联同样在肌动蛋白融合焦点富集,进而协同促进细胞融合。WEICHERT等[41]发现麦角甾醇前体与脂肪侧链共轭双键可干扰丝裂原活化蛋白激酶信号级联,影响细胞融合信号传导,破坏细胞通讯协调,抑制细胞融合。ACQUISTAPACE等[42]发现小鼠心肌细胞与人脂肪间充质干细胞共培养时发生异种细胞融合,人脂肪间充质干细胞线粒体可转移到小鼠心肌细胞并参与其能量代谢。WADA等[43]发现在细胞融合过程中利用微流控技术可以定量控制线粒体转移,甚至实现单个线粒体转移,可以精确调节细胞异质性。国内学者认为细胞融合时伴发线粒体挤出可实现细胞之间线粒体转移[44]。以上研究说明,成体细胞融合也受到细胞动力学相关蛋白调控,丝裂原活化蛋白激酶信号通路在细胞融合启动过程中发挥了重要作用。在成体细胞融合过程中,供体细胞线粒体可转移到宿主细胞并参与其能量代谢,而微流控技术可定量控制线粒体转移数量。
总之,细胞之间线粒体转移可通过细胞融合实现,但细胞动力学相关蛋白和丝裂原活化蛋白激酶信号通路调控细胞融合时可发生多种胞质成分转移,其中线粒体转移对融合细胞的影响尚不明确,有待进一步深入研究。
文章总结了细胞接触状态下线粒体转移的作用及分子调控机制,见表 1。

2.2 细胞非接触状态下线粒体转移的作用及分子调控机制 在体内外环境中,非接触状态下的细胞之间同样存在线粒体转移现象。在非接触状态下,供体细胞可以产生胞外线粒体,依靠微囊泡将线粒体转移到宿主细胞并调控其生物学行为,而微囊泡转运过程受到细胞骨架蛋白、小分子GTP结合蛋白及锚定蛋白等因素的影响。
3.2.1 胞外线粒体的形成、转移及生理作用 胞外囊泡是一种可以包裹蛋白质、脂质和核酸等生物大分子或颗粒物质,并在细胞间转移而不被降解的稳定膜泡结构,主要由细胞(器)膜外凸或内凹形成,可分为凋亡小体(800-5 000 nm)、微囊泡(150-1 000 nm)和外泌体(30-150 nm),其中细胞质膜出芽形成的微囊泡可以包裹线粒体并将其分泌出胞,形成胞外线粒体[45-48]。微囊泡将线粒体转移到宿主细胞后,可以提高宿主细胞增殖活力,减轻氧化应激损伤,抑制细胞衰老凋亡,同时可以介导炎症信号传导,发挥免疫调节作用[49-54]。
许多研究发现干细胞、免疫细胞和血小板等都可以释放包裹线粒体的微囊泡(microparticles contained mitochondria,mitoMPs)。在血细胞研究领域,BOUDREAU等[53]发现活化的血小板可以释放mitoMPs和无囊泡包裹的游离线粒体。WANG等[54]发现血小板线粒体可以调控血小板激活信号影响凝血级联反应,还可以产生氧自由基促进血栓形成。文章推测临床工作中利用富血小板血浆治疗多种组织损伤,其作用原理可能与血小板线粒体功能相关[55]。在免疫细胞研究领域,GAO等[56]发现M1巨噬细胞的炎性线粒体可以通过微囊泡转移到胰腺β细胞并诱导其凋亡。PUHM等[57]发现活化的单核细胞通过微囊泡释放应激线粒体可诱导内皮细胞发生炎症反应,而正常线粒体不会诱发明显炎症反应。以上研究说明,在线粒体移植疗法治疗疾病的过程中,维持供体细胞线粒体质量应该是决定治疗效果的关键。
在干细胞研究领域,IKEDA等[58]发现来源于干细胞的胞外线粒体可以转移到心肌细胞并改善其能量合成,体内注射干细胞线粒体可有效提高缺血心肌的能量合成水平,提示胞外线粒体转移对宿主细胞生物学行为的影响可能是干细胞发挥远程调控作用的重要机制。在呼吸道上皮细胞研究领域,HOUGH等[59]发现从呼吸道上皮细胞中分离的包裹线粒体的微囊泡可以调控细胞内钙离子水平,进而影响气道收缩力,提示包裹线粒体的微囊泡在细胞之间的转移可能改变组织周围的生物力学环境。 在泌尿系统细胞研究领域,DOULAMIS等[60]发现经动脉注射自身无囊泡包裹的游离线粒体对猪肾缺血再灌注损伤具有保护作用,显著增强肾功能,减轻肾脏细胞损害,提示通过人工干预实现细胞非接触状态下的线粒体转移同样可以发挥线粒体保护作用。另外,有些学者认为干细胞通过微囊泡将线粒体转移到免疫细胞可显著增强免疫细胞吞噬活性,发挥免疫调控作用;干细胞通过微囊泡将线粒体转移到损伤的宿主细胞,可有效改善宿主细胞的线粒体功能,减轻组织损伤[61]。近些年,有研究发现在脑损伤小鼠的外周血和蛛网膜下腔出血大鼠的脑脊液中存在无囊泡包裹的游离线粒体,但无囊泡包裹的游离线粒体的形成、转移及生理作用尚不清楚,值得进一步研究[62-63]。
总之,多种细胞可以通过微囊泡将自身线粒体释放出胞形成游离线粒体,而胞外线粒体被宿主细胞摄取后融入其线粒体网络,可影响宿主细胞的生物学行为。因此,微囊泡转移途径使线粒体供体细胞能够发挥远距离调控宿主细胞生物学行为的作用。
2.2.2 线粒体出胞的分子调控机制 真核细胞可以释放胞外线粒体,而线粒体出胞过程作为非接触状态下细胞之间线粒体转移的开端,其分子调控机制尚不完全清楚。在血细胞研究领域,BOUDREAU等[45]发现血小板释放包裹线粒体的微囊泡和无囊泡包裹的游离线粒体受到肌动蛋白直接调控,这与真核细胞中微管-肌动蛋白骨架调控细胞器转运的方式存在差异。以上研究提示人体细胞释放线粒体受到细胞骨架系统和细胞动力学相关蛋白的影响。在肿瘤细胞研究领域,GRIGORIEV等[64]发现小分子GTP结合蛋白家族成员6参与宫颈癌细胞囊泡胞吐过程;小分子GTP结合蛋白家族成员6与微管和驱动蛋白1协同,将囊泡转移到胞外;小分子GTP结合蛋白家族成员6引导囊泡聚集在富含皮质蛋白的质膜位点,促进细胞完成胞吐。因此,小分子GTP结合蛋白家族成员6可能参与调控细胞线粒体的出胞过程。
在衰老细胞研究领域,CHOI等[65]发现衰老细胞囊泡和质膜上的可溶性N-乙基顺丁烯二酰亚胺敏感融合蛋白受体(soluble N-ethyl-maleimide sensitive factor attachment protein receptors,SNAREs)影响囊泡与质膜的锚定过程,而线粒体磷脂酶D(mitochondrial Phospholipase D, MitoPLD)通过调控脂质环境影响囊泡与细胞质膜的融合,提示可溶性N-乙基顺丁烯二酰亚胺敏感融合蛋白受体和线粒体磷脂酶D可能影响微囊泡介导的线粒体出胞过程。在干细胞研究领域,PHINNEY等[66]发现骨髓间充质干细胞释放的包裹线粒体的微囊泡表达囊泡转运所需的肿瘤抑制基因101(tumor suppressor gene 101,TSG101)相关的内涵体分选复合物(endosomal sorting complex,ESCRT)和抑制蛋白结构域蛋白1 (arrestin domain-containing protein 1,ARRDC1),提示内涵体分选复合物和抑制蛋白结构域蛋白1可能参与调控干细胞线粒体出胞,进而影响干细胞的远程调控功能。在神经细胞研究领域,HAYAKAWA等[67]发现星形胶质细胞释放线粒体受到钙依赖的环状ADP核糖水解酶和环状ADP核糖信号通路影响,提示环状ADP核糖水解酶和环状ADP核糖参与调控线粒体出胞过程。近些年,有研究者认为清除受损线粒体才能维持细胞内线粒体网络的正常运行,受损线粒体出胞是维持细胞膜电位和细胞活力所必需的,也是细胞线粒体质量控制的重要途径[68]。
总之,线粒体出胞既是供体细胞发挥远程调控作用的关键,也是氧化应激损伤细胞进行自我保护的方式。另外,在探究干细胞线粒体能否改善宿主细胞线粒体功能时,应当注意检测干细胞的氧化应激水平,避免发生功能障碍的线粒体对实验造成不良影响。
2.2.3 线粒体入胞的分子调控机制 供体细胞通过微囊泡转移线粒体必然经历宿主细胞内吞线粒体的过程,继而实现线粒体入胞并完成线粒体网络整合,最终发挥线粒体转移的作用。细胞内吞作用是细胞质膜变形将胞外物质转移入胞的过程,包括吞噬作用(摄入直径> 1 μm的颗粒物质)、胞饮作用(摄入多数直径< 1 μm溶质或液体)、受体介导的内吞作用(细胞依靠细胞表面的受体特异性地摄取细胞外蛋白或其他化合物)。真核细胞可以内吞胞外线粒体,并且有研究者发现真核细胞通过胞饮作用摄取胞外线粒体[69-70]。胞饮作用是液态物质进入细胞的主动运输形式,根据其产生机制可分为 4种:网格蛋白依赖的内吞、陷穴蛋白依赖的内吞、巨胞饮、网格蛋白和陷穴蛋白非依赖的内吞。然而,线粒体入胞的分子调控机制尚不完全清楚。在心血管细胞研究领域,KITANI等[71]发现异丙基阿米洛利乙酯(ethylisopropyl amiloride,EIPA)作为胞饮特异性抑制剂可以阻断线粒体转移对心肌成纤维细胞的治疗作用,提示胞外线粒体入胞过程与宿主细胞的胞饮作用相关。巨胞饮是依赖肌动蛋白的非受体内吞途径,发生过程中细胞膜出现皱褶并形成直径为0.2-5.0 μm的巨胞饮体,非选择性地内吞细胞外营养物质和液相大分子。KESNER等[72]测定血管内皮细胞巨胞饮和内吞作用抑制剂对线粒体转移的影响,发现细胞松弛素D(肌动蛋白聚合抑制剂)和诺考唑(微管组装抑制剂)可以抑制线粒体转移,而氯丙嗪(网格蛋白介导的抑制剂)不能减少线粒体转移,提示细胞不是通过网格蛋白依赖的内吞作用获得外源性线粒体,而是依靠肌动蛋白和微管介导的巨胞饮。在免疫细胞研究领域,PACAK等[73]发现巨噬细胞与细胞松弛素D预孵育可明显抑制肌动蛋白依赖的内吞作用,而激活肌动蛋白依赖的内吞作用可促进线粒体内化,提示细胞动力学相关蛋白参与调控线粒体入胞过程。在肿瘤细胞研究领域,SUN等[74]发现胶质瘤细胞通过内吞作用获得外源性线粒体,而NAD+-CD38-cADPR-Ca2+信号通路调控细胞骨架重塑和细胞质膜内陷,进而影响线粒体入胞,提示NAD+-CD38-cADPR-Ca2+信号通路在线粒体入胞过程中发挥调控作用。
总之,微囊泡转移线粒体入胞是宿主细胞摄取胞外线粒体的关键步骤,受到细胞动力学相关蛋白、细胞骨架系统及细胞信号传导通路等因素的影响;调控线粒体入胞过程可影响细胞之间线粒体转移的效率,进而影响宿主细胞命运。因此,研究微囊泡转移途径可为探究非接触状态下细胞之间线粒体转移调控机制提供新路径,并为增强线粒体移植疗效提供新策略。
文章总结了细胞非接触状态下线粒体转移的作用及分子调控机制,见表2。

| [1] HARRINGTON JS, RYTER SW, PLATAKI M, et al. Mitochondria in health, disease, and aging. Physiol Rev. 2023;103(4):2349-2422. [2] DING X, ZHANG Y, PAN PC, et al. Multiple mitochondria-targeted components screened from Sini decoction improved cardiac energetics and mitochondrial dysfunction to attenuate doxorubicin-induced cardiomyopathy. Theranostics. 2023;13(2):510-530. [3] VELARDE F, EZQUERRA S, DELBRUYERE X, et al. Mesenchymal stem cell-mediated transfer of mitochondria: mechanisms and functional impact. Cell Mol Life Sci. 2022;79(3):177. [4] MALEKPOUR K, HAZRATI A, SOUDI S, et al. Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery. J Control Release. 2023;354:755-769. [5] WEI B, JI ML, Lin YC, et al. Mitochondrial transfer from bone mesenchymal stem cells protects against tendinopathy both in vitro and in vivo. Stem Cell Res Ther. 2023;14(1):104. [6] ZHANG HY, YU XX, YE JF, et al. Systematic investigation of mitochondrial transfer between cancer cells and T cells at single-cell resolution. Cancer Cell. 2023;41(10):1788-1802. [7] GOLIWAS KF, LIBRING S, BERESTESKY E, et al. Mitochondrial transfer from cancer-associated fibroblasts increases migration in aggressive breast cancer. J Cell Sci. 2023;136(14):jcs260419. [8] SUBRAMANIAM MD, CHIRAYATH RB, IYER M, et al. Mesenchymal stem cells (MSCs) in Leber’s hereditary optic neuropathy (LHON): a potential therapeutic approach for future. Int Ophthalmol. 2022;42(9): 2949-2964. [9] AHMAD T, MUKHERJEE S, PATTNAIK B, et al. Miro1 regulatesintercellular mitochondrial transport enhances mesenchymalstem cell rescue efficacy. EMBOJ. 2014;33(9):994-1010. [10] ISLAM MN, DAS SR, EMIN MT, et al. Mitochondrial transfer from bone marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. [11] YANG YH, ZHANG Y, LI WJ, et al. Spectraplakin induces positive feedback between fusogens and the actin cytoskeleton to promote cell-cell fusion. Dev cell. 2017;41(1):107-120. [12] WANG J, LI H, YAO Y, et al. Stem cell-derived mitochondria transplantation: a novel strategy and the challenges for the treatment of tissue injury. Stem Cell Res Ther. 2018;9(1):106. [13] MELWANI PK, PANDEY BN. Tunneling nanotubes: The intercellular conduits contributing to cancer pathogenesis and its therapy. Biochim Biophys Acta Rev Cancer. 2023;1878(6):189028. [14] ZHANG F, ZHENG XL, ZHAO FZ, et al. TFAM-Mediated mitochondrial transfer of MSCs improved the permeability barrier in sepsis-associated acute lung injury. Apoptosis. 2023;28(7-8):1048-1059. [15] LIN RZ, IM GB, LUO AC, et al. Mitochondrial transfer mediates endothelial cell engraftment through mitophagy. Nature. 2024; 629(8012):660-668. [16] JIANG D, CHEN FX, ZHOU H, et al. Bioenergetic crosstalk between mesenchymal stem cells and various ocular cells through the intercellular trafficking of mitochondria. Theranostics. 2020;10(16): 7260-7272. [17] SAGAR S, FAIZAN MI, CHAUDHARY N, et al. Obesity impairs cardiolipin- dependent mitophagy and therapeutic intercellular mitochondrial transfer ability of mesenchymal stem cells. Cell Death Dis. 2023;14(5):324. [18] 杨焜,程意,郭雪君.隧道纳米管介导线粒体转移缓解氧化应激及其在慢性阻塞性肺疾病的研究进展[J].临床肺科杂志,2024, 29(1):65-68. [19] AMIN R, RAINER S, IVANKA M, et al. Nanotubular highways for intercellular organelle transport.Science. 2004;303(5660):1007-1010. [20] WANG X, GERDES H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22(7):1181-1191. [21] WANG JC, LIU X, QIU Y, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11(1):11. [22] TISHCHENKO A, AZORIN DD, BRIME LV, et al. Cx43 and associated cell signaling pathways regulate tunneling nanotubes in breast cancer cells. Cancers (Basel). 2020;12(10):2798. [23] KOYANAGI M, BRANDES RP, HAENDELER J, et al. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96(10): 1039-1041. [24] SAITO K, ZHANG Q, YANG H, et al. Exogenous mitochondrial transfer and endogenous mitochondrial fission facilitate AML resistance to OxPhos inhibition. Blood Adv. 2021;5(20):4233-4255. [25] BURT R, DEY A, AREF S, et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 2019;134(17):1415-1429. [26] BOISE LH, SHANMUGAM M. Stromal support of metabolic function through mitochondrial transfer in multiple myeloma. Cancer Res. 2019;79(9):2102-2103. [27] NI XC, WANG HF, CAI YY, et al. Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol. 2022;54:102363. [28] LEIGHTON SE, WONG RS, LUCACIU SA, et al. Cx31.1 can selectively intermix with co-expressed connexins to facilitate its assembly into gap junctions. J Cell Sci. 2024;137(7):jcs261631. [29] ZAMPIERI LX, ALMEIDA CS, RONDEAU JD, et al. Mitochondrial transfer in cancer: a comprehensive review. Int J Mol Sci. 2021;22(6):3245. [30] SINGH AK, CANCELAS JA. Gap junctions in the bone marrow lympho-hematopoietic stem cell niche, leukemia progression, and chemoresistance. Int J Mol Sci. 2020;21(3):796. [31] LI HYZ, WANG C, HE T, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9(7):2017-2035. [32] ANDREW SK, TERASE YS, ANTHONY HPM, et al. Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung.Stem Cell Res Ther.2016;7(1):91. [33] 诸葛晓萱,李策,包广洁,等.缝隙连接蛋白43经典与非经典作用在疾病治疗中的潜在价值[J].中国组织工程研究,2024,27(7): 1130-1136. [34] LIU ZH, SUN Y, QI ZT, et al. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 2022;12(1):66. [35] NORRIS RP. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic. 2021;22(6):174-179. [36] IOSILEVSKII Y, PODBILEWICZ B. Programmed cell fusion in development and homeostasis. Curr Top Dev Biol. 2021;144:215-244. [37] WHITLOCK JM, CHERNOMORDIK LV. Flagging fusion: Phosphatidylserine signaling in cell-cell fusion. J Biol Chem. 2021; 296:100411. [38] SMUROVA K, PODBILEWICZ B. RAB-5- and DYNAMIN-1-mediated endocytosis of EFF-1 fusogen controls cell-cell fusion. Cell Rep. 2016; 14(6):1517-1527. [39] MERLINI L, KHALILI B, DUDIN O, et al. Inhibition of Ras activity coordinates cell fusion with cell-cell contact during yeast mating. J Cell Biol. 2018;217(4):1467-1483. [40] DUDIN O, MERLINI L, MARTIN SG. Spatial focalization of pheromone/MAPK signaling triggers commitment to cell–cell fusion. Gene Dev. 2016;30(19):2226-2239. [41] WEICHERT M, LICHIUS A, PRIEGNITZ BE, et al. Accumulation of specific sterol precursors targets a MAP kinase cascade mediating cell–cell recognition and fusion. PNAS. 2016;113(42): 11877-11882. [42] ACQUISTAPACE A, BRU T, LESAULT PF, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29(5):812-824. [43] WADA KI, HOSOKAWA K, ITO Y, et al. Quantitatively controlled intercellular mitochondrial transfer by cell fusion-based method using a microfluidic device. Methods Mol Biol. 2021;2277:39-47. [44] 肖雨倩,白艳杰,王岩,等.线粒体转移在脑卒中后认知障碍中的研究进展[J].中国全科医学,2023,26(30):3833-3840. [45] BOUDREAU LH, DUCHEZ AC, CLOUTIER N, et al. Platelets release mitochondria serving as substrate for bactericidalgroup IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14): 2173-2183. [46] SOUZA AD, BURCH A, DAVE KM, et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J Control Release. 2021;338:505-526. [47] GEORGATZAKOU HT, FORTIS SP, PAPAGEORGIOU EG, et al. Blood cell-derived microvesicles in hematological diseases and beyond. Biomolecules. 2022;12(6):803. [48] MAACHA S, BHAT AA, JIMENEZ L, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18(1):1-16. [49] VIJAYAN V, YAN H, LOHMEYER JK, et al. Extracellular release of mitochondria induced by pre-hematopoietic stem cell transplant conditioning exacerbates GVHD. Blood Adv. 2024;3:2023012328. [50] ZOROVA LD, KOVALCHUK SI, POPKOV VA, et al. Do extracellular vesicles derived from mesenchymal stem cells contain functional mitochondria? Int J Mol Sci. 2022;23(13):7408. [51] SCANDELLA V, PETRELLI F, MOORE DL, et al. Neural stem cell metabolism revisited: a critical role for mitochondria. Trends Endocrinol Metab. 2023;34(8):446-461. [52] MALEKPOUR K, HAZRATI A, SOUDI S, et al. Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery. J Control Release. 2023;354:755-769. [53] BOUDREAU LH, DUCHEZ AC, CLOUTIER N, et al. Platelets release mitochondria serving as substrate for bactericidalgroup IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14): 2173-2183. [54] WANG L, WU Q, FAN ZJ, et al. Platelet mitochondrial dysfunction and the correlation with humandiseases. Biochem Soc T. 2017;45(6): 1213-1223. [55] MCCARREL TM. Equine platelet-rich plasma. Vet Clin North Am Equine Pract. 2023;39(3):429-442. [56] GAO YH, MI NN, WU WX, et al. Transfer of inflammatory mitochondria via extracellular vesicles from M1 macrophages induces ferroptosis of pancreatic beta cells in acute pancreatitis. J Extracell Vesicles. 2024;13(2):e12410. [57] PUHM F, AFONYUSHKIN T, RESCH U, et al. Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circ Res. 2019;125(1): 43-52. [58] IKEDA G, SANTOSO MR, TADA Y, et al. Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J Am Coll Cardiol. 2021;77(8): 1073-1088. [59] HOUGH KP, TREVOR JL, STRENKOWSKI JG, et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Bio. 2018;18:54-64. [60] DOULAMIS IP, GUARIENTO A, DUIGNAN T, et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am J Physiol Renal Physiol. 2020;319(3):F403-F413. [61] 周健,高俊杰,张长青.线粒体转移与骨和软骨[J].国际骨科学杂志,2024,45(2):53-56. [62] ZHAO ZL, ZHOU Y, HILTON T, et al. Extracellular mitochondria released from traumatized brains induced platelet procoagulant activity. Haematologica. 2020;105(1):209-217. [63] CHOU SHY, LAN J, ELGA E, et al. Extracellular mitochondria in cerebrospinal fluid and neurological recovery after subarachnoid hemorrhage. Stroke. 2017;48(8):2231-2237. [64] GRIGORIEV I, SPLINTER D, KEIJZER N, et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13(2):305-314. [65] CHOI SY, HUANG P, JENKINS GM, et al. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8(11):1255-1262. [66] PHINNEY DG, DI GM, NJAH J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6(1):8472. [67] HAYAKAWA K, ESPOSITO E, WANG X, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613): 551-555. [68] JIAO HF, JIANG D, HU XY, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell. 2021;184(11): 2896-2910. [69] CHEN J, FU CY, SHEN GR, et al. Macrophages induce cardiomyocyte ferroptosis via mitochondrial transfer. Free Radic Biol Med. 2022;190: 1-14. [70] RENNICK JJ, JOHNSTON APR, PARTON RG. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol. 2021;16(3):266-276. [71] KITANI T, KAMI D, MATOBA S, et al. Internalization of isolated functional mitochondria: involvement of macropinocytosis. J Cell Mol Med. 2014;18(8):1694-1703. [72] KESNER EE, SAADA RA, LORBERBOUM GH. Characteristics of mitochondrial transformation into human cells. Sci Rep. 2016;6(1): 26057. [73] PACAK CA, PREBLE JM, KONDO H, et al. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open. 2015;4(5):622-626. [74] SUN C, LIU XX, WANG B, et al. Endocytosis-mediated mitochondrial transplantation: transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics. 2019;9(12):3595-3607. |
| [1] | 赵济宇, 王少伟. 叉头框转录因子O1信号通路与骨代谢[J]. 中国组织工程研究, 2025, 29(9): 1923-1930. |
| [2] | 袁维勃, 刘 婵, 余丽梅. 肝脏类器官在肝脏疾病模型与移植治疗中的应用潜力[J]. 中国组织工程研究, 2025, 29(8): 1684-1692. |
| [3] | 喻 婷, 吕冬梅, 邓 浩, 孙 涛, 程 钎. 淫羊藿苷预处理增强人牙周膜干细胞对M1型巨噬细胞的影响[J]. 中国组织工程研究, 2025, 29(7): 1328-1335. |
| [4] | 杨治航, 孙祖延, 黄文良, 万 喻, 陈仕达, 邓 江. 神经生长因子促进兔骨髓间充质干细胞软骨分化并抑制肥大分化[J]. 中国组织工程研究, 2025, 29(7): 1336-1342. |
| [5] | 胡涛涛, 刘 兵, 陈 诚, 殷宗银, 阚道洪, 倪 杰, 叶凌霄, 郑祥兵, 严 敏, 邹 勇. 过表达神经调节蛋白1的人羊膜间充质干细胞促进小鼠皮肤创面愈合[J]. 中国组织工程研究, 2025, 29(7): 1343-1349. |
| [6] | 金 凯, 唐 婷, 李美乐, 谢裕安. 人脐带间充质干细胞条件培养基及外泌体对肝癌细胞增殖、迁移、侵袭和凋亡的影响[J]. 中国组织工程研究, 2025, 29(7): 1350-1355. |
| [7] | 李帝均, 酒精卫, 刘海峰, 闫 磊, 李松岩, 王 斌. 明胶三维微球装载人脐带间充质干细胞修复慢性肌腱病[J]. 中国组织工程研究, 2025, 29(7): 1356-1362. |
| [8] | 娄 国, 张 敏, 付常喜. 8周运动预适应增强脂肪干细胞治疗心肌梗死大鼠的效果[J]. 中国组织工程研究, 2025, 29(7): 1363-1370. |
| [9] | 刘 琪, 李林臻, 李玉生, 焦泓焯, 杨 程, 张君涛. 淫羊藿苷含药血清促进3种细胞共培养体系中软骨细胞增殖和干细胞成软骨分化[J]. 中国组织工程研究, 2025, 29(7): 1371-1379. |
| [10] | 艾克帕尔·艾尔肯, 陈晓涛, 吾凡别克·巴合提. 成骨诱导人牙周膜干细胞来源外泌体促进炎症微环境下人牙周膜干细胞成骨分化[J]. 中国组织工程研究, 2025, 29(7): 1388-1394. |
| [11] | 章镇宇, 梁秋健, 杨 军, 韦相宇, 蒋 捷, 黄林科, 谭 桢. 新橙皮苷治疗骨质疏松症的靶点及对骨髓间充质干细胞成骨分化的作用[J]. 中国组织工程研究, 2025, 29(7): 1437-1447. |
| [12] | 吕丽婷, 于 霞, 张金梅, 高巧婧, 刘仁凡, 李 梦, 王 璐. 脑衰老与外泌体研究进程及现状的文献计量学分析[J]. 中国组织工程研究, 2025, 29(7): 1457-1465. |
| [13] | 谢刘刚, 崔书克, 郭楠楠, 李遨宇, 张菁瑞. 干细胞治疗阿尔茨海默病的研究热点与前沿[J]. 中国组织工程研究, 2025, 29(7): 1475-1485. |
| [14] | 李佳林, 张耀东, 娄艳茹, 于 洋, 杨 蕊. 间充质干细胞分泌组发挥作用的分子机制[J]. 中国组织工程研究, 2025, 29(7): 1512-1522. |
| [15] | 孙玉婷, 吴家媛, 张 剑. 影响牙髓干细胞成骨及成牙本质分化的相关物理因素及作用机制[J]. 中国组织工程研究, 2025, 29(7): 1531-1540. |
有研究发现,来源于干细胞的线粒体可通过多种途径转移到受损宿主细胞并与其线粒体网络融合,可有效改善宿主细胞的线粒体功能,有助于修复组织损伤[3-4]。有学者深入研究发现,转移到受损宿主细胞的外源性线粒体可以替换宿主细胞功能障碍的线粒体,降低胞内氧化应激水平,防止细胞损伤加重[5]。然而,肿瘤细胞接受外源性线粒体后,其侵袭力和药物敏感性可发生明显增强或减弱[6-7]。正常细胞接受肿瘤细胞的线粒体后,其活性氧水平明显升高,造成机体组织氧化应激损伤。因此,细胞之间线粒体转移对疾病发生发展的影响具有两面性,值得深入探讨。
线粒体可通过隧道纳米管(tunneling nanotubes,TNTs)、缝隙连接(gap junction,GJ)、细胞融合(cell fusion,CF)及微囊泡(microvesicles,MVs)等途径进行细胞间转移,但线粒体转移的分子调控机制尚不完全清楚[8]。有研究发现线粒体Rho GTP酶1(mitochondrial Rho GTPase 1,Miro1)、缝隙连接蛋白43(connexin43,Cx43)、微丝骨架、融合蛋白、环状ADP核糖水解酶(cyclic ADP ribose hydrolase,CD38)等参与调控细胞间线粒体转移[9-12]。然而,目前学者们对细胞间线粒体转移的分子调控机制尚未达成共识。
虽然许多研究已经对线粒体功能、线粒体转移途径及线粒体转移的生理作用进行过详细论述,但却忽视了线粒体转移调控机制这一关键因素在线粒体转移治疗线粒体相关疾病中的作用。文章就细胞间线粒体转移的作用及调控机制作一综述,以期为调控线粒体转移在组织工程和线粒体相关疾病防治中的应用提供新的思路。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者在 2024年1月进行文献检索。
1.1.2 检索文献时限 文献重点检索时间范围为2014年1月至2024年1月,同时纳入少量远期经典文献。
1.1.3 检索数据库 中国知网和PubMed数据库。
1.1.4 检索词 以“线粒体转移,隧道纳米管,缝隙连接,微囊泡,细胞融合”和“mitochondrial transfer,tunneling nanotubes,gap junctions,microvesicles,cell fusion”分别为中、英文检索词进行检索。
1.1.5 检索文献类型 研究原著、综述和荟萃分析。
1.1.6 手工检索情况 无。
1.1.7 检索策略 以 PubMed 数据库检索策略为例,见图1。
1.1.8 检索文献量 计算机初检得到中国知网数据库中文文献307篇,PubMed数据库英文文献419篇。
1.2 入组标准
1.2.1 纳入标准 ①关于细胞之间线粒体转移作用的文献;②与隧道纳米管、缝隙连接、微囊泡及细胞融合转移线粒体相关的论著和综述;③具有创新性的文章。
1.2.2 排除标准 ①与研究内容不相关的文献;②内容重复性文献;③ 陈旧无参考价值的文献。
1.3 文献质量评估和数据的提取 经资料收集者互相评估纳入文献的有效性和适用性,通过阅读文题和摘要进行初步筛选;排除中英文文献重复性研究、内容不相关的文献;最后纳入74篇文献进行综述,其中来源于中国知网数据库中文文献4篇,PubMed数据库英文文献70篇。文献筛选流程见图2。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
在细胞接触状态下,供体细胞可以通过隧道纳米管、缝隙连接、细胞融合将自身线粒体转移到宿主细胞。在细胞非接触状态下,供体细胞可以通过微囊泡将自身线粒体转移到宿主细胞,也可直接释放无囊泡包裹的线粒体进行细胞之间的线粒体转移。
在细胞接触状态下,供体细胞将线粒体转移到损伤的宿主细胞,可以有效改善损伤细胞的线粒体功能并促进组织再生,降低胞内氧化应激水平,抑制损伤细胞衰老凋亡,改善细胞的通透性并减少渗出,减轻炎症反应,降低炎性因子分泌水平,有利于维持机体内环境稳定。损伤细胞也可将自身功能障碍的线粒体转移到间充质干细胞,减少自身氧化应激损伤。然而,供体细胞同样可以将线粒体转移到肿瘤细胞,增强肿瘤细胞迁移和增殖能力,提高肿瘤细胞的能量合成水平,减轻肿瘤细胞的氧化应激损伤,从而对机体造成不良影响。因此,线粒体转移对疾病的发生发展具有双向作用,摸索线粒体转移的适用条件至关重要。在细胞非接触状态下,正常供体细胞将自身线粒体转移到损伤的宿主细胞,可以改善宿主细胞的线粒体功能,提高宿主细胞增殖活力,减轻氧化应激损伤,抑制宿主细胞衰老凋亡,影响炎症信号传导发挥免疫调节作用,调控血小板激活影响凝血级联反应,调控胞内钙离子水平影响平滑肌收缩。然而,损伤的供体细胞同样可以将应激线粒体转移到宿主细胞,诱发细胞炎症反应,导致宿主细胞衰老凋亡,从而加重组织损伤。
文章总结了细胞发生线粒体功能障碍对机体的生理影响,见表3。
细胞通过隧道纳米管转移线粒体时,线粒体Rho GTP酶1促进隧道纳米管形成,并将驱动蛋白家族成员5与线粒体连接,启动线粒体转移。氧化应激环境促使线粒体从正常细胞转移到应激损伤细胞,且线粒体转移效率与隧道纳米管数量呈正相关。缝隙连接蛋白43参与调控隧道纳米管的形成,其表达缺失可引起隧道纳米管长度和数量减少。供体细胞通过隧道纳米管转移线粒体依赖宿主细胞表面环状ADP核糖水解酶的表达。细胞通过缝隙连接转移线粒体时,缝隙连接蛋白43影响缝隙连接形成,且缝隙连接具有钙离子依赖性。全反式维甲酸(缝隙连接增效剂)促进线粒体转移,18β-甘草次酸(缝隙连接抑制剂)抑制线粒体转移。供体细胞表面缝隙连接蛋白43和宿主细胞表面缝隙连接蛋白32形成异型缝隙连接参与线粒体转移。部分缝隙连接形成可以不依赖缝隙连接蛋白43,且间充质干细胞通过缝隙连接转移线粒体具有单向性。 细胞通过细胞融合转移线粒体时,线虫发育融合蛋白1通过肌动蛋白结合蛋白与纤维状肌动蛋白细胞骨架连接,肌动蛋白招募融合素到细胞融合位点,且线虫发育融合蛋白1促进肌动蛋白结合蛋白与纤维状肌动蛋白结合,有利于细胞融合。磷脂酰丝氨酸和磷脂酰丝氨酸识别蛋白也参与细胞融合过程。 G蛋白偶联受体、大鼠肉瘤蛋白和丝裂原激活蛋白激酶组成的信号通路参与调控细胞融合过程,激活大鼠肉瘤蛋白1可促进细胞融合。信息素、信息素受体和丝裂原激活蛋白激酶信号级联在肌动蛋白融合焦点富集,协同促进细胞融合。麦角甾醇前体与脂肪侧链共轭双键干扰丝裂原激活蛋白激酶信号级联,可抑制细胞融合。细胞顶膜区域富集线虫发育融合蛋白1的位置可发生质膜融合,而下调动力蛋白和小分子GTP结合蛋白家族成员5表达水平会导致细胞过度融合。细胞融合时使用微流控技术可以实现定量控制线粒体转移。细胞通过微囊泡转移线粒体时,微管-肌动蛋白骨架参与调控线粒体转运。小分子GTP结合蛋白家族成员6与微管和驱动蛋白1协同将囊泡转移到胞外,并引导囊泡聚集在富含皮质蛋白的质膜位点,促进细胞完成胞吐,可能有利于线粒体出胞。包裹线粒体的微囊泡表达囊泡转运所需的内涵体分选复合物和抑制蛋白结构域蛋白1,提示内涵体分选复合物和抑制蛋白结构域蛋白1参与调控线粒体转移。环状ADP核糖水解酶和环状ADP核糖影响细胞释放线粒体,参与调控线粒体出胞过程。可溶性N-乙基顺丁烯二酰亚胺敏感融合蛋白受体影响囊泡与质膜的锚定过程,而线粒体磷脂酶D通过调控脂质环境影响囊泡与细胞质膜的融合,提示可溶性N-乙基顺丁烯二酰亚胺敏感融合蛋白受体和线粒体磷脂酶D可能影响线粒体转移。胞饮特异性抑制剂异丙基阿米洛利乙酯阻断线粒体转移,提示胞外线粒体转移与胞饮作用相关。细胞松弛素D(肌动蛋白聚合抑制剂)和诺考唑(微管组装抑制剂)可以抑制线粒体转移,而氯丙嗪(网格蛋白介导的抑制剂)不能减少线粒体转移,提示宿主细胞获取外源性线粒体是依靠肌动蛋白和微管介导的巨胞饮。NAD+-CD38-cADPR-Ca2+ 信号通路调控细胞骨架重塑和细胞质膜内陷,可影响线粒体转移。总之,细胞之间线粒体转移是在细胞信号传导蛋白、细胞动力学相关蛋白及氧化应激环境的影响下实现的,通过调控线粒体转移治疗线粒体相关疾病需要根据以上影响因素制定策略。
然而,以上研究存在一些问题:①文章中部分研究只观察了细胞之间线粒体转移的现象,并未深入讨论线粒体转移的作用,如抑制氧化应激、提高能量代谢及参与免疫调控等,并且还需要进一步探讨细胞之间线粒体转移的调控机制。②虽然细胞之间线粒体转移在调控机体生理活动过程中发挥重要作用,但在细胞接触状态下的线粒体转移伴随着多种胞内成分的同步转移,而在细胞非接触状态下的线粒体转移,微囊泡包裹线粒体的同时不可避免的携带其他胞内成分。然而,大多数基础研究论证线粒体转移的作用时没有适当屏蔽其他胞内成分的干扰。部分研究判断线粒体转移的作用时仅依靠线粒体示踪和能量合成检测结果是不充分的,在评价细胞之间线粒体转移的作用时应准备多方面论据。③无囊泡包裹的胞外线粒体也参与细胞之间线粒体转移,但缺乏相关研究论证其生理作用和转移调控机制,有必要进行深入探讨。④大多数研究支持供体细胞单向转移线粒体到损伤宿主细胞,有效改善宿主细胞线粒体功能,发挥细胞保护作用。然而,有一部分研究发现损伤供体细胞转移线粒体到宿主细胞,避免自身发生氧化应激损伤,发挥自我保护作用。以上线粒体转移相关研究的结论存在明显差异,将来需要对线粒体转移的双向作用进行深入研究。⑤关于细胞之间线粒体转移调控机制的研究较多,但对细胞融合时线粒体转移的研究不够充分。⑥目前缺乏调控细胞之间线粒体转移治疗疾病的临床研究以供参考。
3.2 作者综述区别于他人他篇的特点 既往研究已对细胞之间线粒体转移的作用和作用机制进行了论述,而且细胞之间线粒体转移的调控机制也被论述过,但尚未对细胞之间线粒体转移的作用和调控机制进行全面综述。文章首次从细胞接触状态和非接触状态两方面综述细胞之间线粒体转移的作用和调控机制。
3.3 综述的局限性 文章将细胞之间线粒体转移的作用和调控机制作为主线,对线粒体转移作用的最新研究报道不够,而且文章主要综述基础研究成果,缺少临床试验结果支持。
3.4 综述的重要意义 细胞之间线粒体转移理论是推动细胞治疗技术的重要基础,但其临床验证结果十分匮乏,主要原因是线粒体移植疗效缺乏说服力,进一步提升疗效是线粒体转移理论应用于临床的关键。促进细胞之间线粒体转移可能是提高细胞治疗疗效的有效措施,但目前缺乏有效药物,文章为促进细胞之间线粒体转移提供参考,为提高细胞治疗疗效和线粒体相关疾病防治提供新思路。同时,线粒体转移本来就是干细胞发挥组织修复作用的重要途径,而干细胞来源的线粒体也具有良好的生物学性能。因此,文章将为干细胞用于组织工程研究提供新思路。
3.5 课题专家组对未来的建议 细胞治疗、线粒体转移和氧化应激是线粒体相关研究领域的热点,细胞之间线粒体转移的作用和调控机制在未来仍将是研究前沿。线粒体转移通常用于心血管系统、泌尿系统、神经系统及呼吸系统疾病相关的动物实验研究,主要通过线粒体局部注射或促进线粒体转移实现修复损伤组织的目标,但对于线粒体注射剂量和潜在风险尚未达成共识,并且缺乏相应的临床验证,值得进一步深入研究。细胞之间线粒体转移是细胞治疗技术的重要理论基础。保持供体细胞线粒体功能并促进细胞之间线粒体转移是影响细胞治疗疗效的重要因素,也是干细胞用于组织工程研究的重要条件。通过调控细胞之间线粒体转移增强细胞治疗疗效的相关研究值得继续推进。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
线粒体:是一种由两层膜包被的半自主细胞器,广泛存在于真核细胞,具有合成能量的功能,是胞内有氧呼吸的主要场所,在细胞生物学行为中发挥重要作用,被称为“细胞动力站”,其直径为0.5-1.0 µm。
线粒体转移:指线粒体通过各种途径进入相邻细胞,或者脱离母细胞形成游离线粒体并前往远处宿主细胞的过程。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
线粒体功能障碍导致细胞衰老凋亡,可加重组织损伤。细胞之间线粒体转移促进损伤细胞恢复线粒体功能,有助于治疗线粒体相关疾病。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||