中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (25): 5469-5477.doi: 10.12307/2025.531
• 干细胞综述 stem cell review • 上一篇 下一篇
主要组织相容性复合体调控帕金森病的免疫反应
关梦雅1,2,任彬彬2,王晶莹1
- 1河南中医药大学康复医学院,河南省郑州市 450046;2河南中医药大学第一附属医院,河南省郑州市 450000
-
收稿日期:2024-05-20接受日期:2024-07-08出版日期:2025-09-08发布日期:2024-12-30 -
通讯作者:任彬彬,博士,硕士研究生导师,主任医师,河南中医药大学第一附属医院,河南省郑州市 450000 -
作者简介:关梦雅,女,1998年生,河南省商丘市人,汉族,硕士研究生,主要从事神经康复方向的研究。 -
基金资助:中医药传承与创新人才工程(仲景工程)(CZ0325-10),项目负责人:任彬彬;2022年度河南省中医药拔尖人才培养项目专项课题(2022ZYBJ09),项目负责人:任彬彬
Major histocompatibility complex regulates immune responses in Parkinson’s disease
Guan Mengya1, 2, Ren Binbin2, Wang Jingying1
- 1School of Rehabilitation, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; 2First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2024-05-20Accepted:2024-07-08Online:2025-09-08Published:2024-12-30 -
Contact:Ren Binbin, MD, Master’s supervisor, Chief physician, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Guan Mengya, Master candidate, School of Rehabilitation, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:Traditional Chinese Medicine Inheritance and Innovation Talent Project (Zhongjing Project), No. CZ0325-10 (to RBB); 2022 Henan Province Traditional Chinese Medicine Top Talent Training Special Project, No. 2022ZYBJ09 (to RBB)
摘要:
文题释义:
帕金森病:作为第二大神经退行性疾病,帕金森病临床表现为多个方面,最主要的是运动症状,如肌强直、运动迟缓、静止性震颤、姿势平衡障碍。除此之外,自主神经功能障碍和精神障碍(便秘、泌尿障碍、睡眠障碍、抑郁、焦虑、认知减退等)也对患者的生活产生严重影响。帕金森病的发病机制尚未完全阐明,多巴胺神经元缺失和α-突触核蛋白的聚集是其主要病理特征。主要组织相容性复合体:人类主要组织相容性复合体也称为人类白细胞抗原系统,分为Ⅰ、Ⅱ和Ⅲ类,它们可以结合来自细胞外或者细胞内的抗原肽,呈递给T细胞,在先天性免疫和适应性免疫反应中发挥不可或缺的作用。
摘要
背景:免疫反应与帕金森病的病理发展密切相关。研究表明,主要组织相容性复合体在免疫应答中发挥关键作用。
目的:综述主要组织相容性复合体调控免疫反应的机制以及对帕金森病病理标志物α-突触核蛋白的影响。
方法:以“Parkinson’s disease,the major histocompatibility complex,innate immunity,adaptive immunity,microglia,T cell,B cell,α-syn,inflammation,MHC-Ⅰ,MHC-Ⅱ,immunotherapy”为检索词在PubMed数据库检索文献,最终纳入92篇文献进行分析。
结果与结论:①先天性免疫反应参与帕金森病的发生发展,小胶质细胞的促炎和抗炎表型的变化可能加剧帕金森病退行性变;②T细胞的表型和功能与帕金森病的进展有关,调节性T细胞促进抗炎小胶质细胞活化,抑制Th亚群;B细胞介导的体液免疫可清除病理性α-突触核蛋白,具体机制需要进一步研究;③主要组织相容性复合体与先天性免疫和适应性免疫的发生密切相关,对帕金森病的炎症产生影响;④α-突触核蛋白调控小胶质细胞的激活和主要组织相容性复合体的表达,导致帕金森病的炎症变化;⑤α-突触核蛋白与帕金森病的免疫反应密切相关,成为治疗帕金森病的重要靶点。
https://orcid.org/0009-0004-3931-4047 (关梦雅)
中图分类号:
引用本文
关梦雅, 任彬彬, 王晶莹. 主要组织相容性复合体调控帕金森病的免疫反应[J]. 中国组织工程研究, 2025, 29(25): 5469-5477.
Guan Mengya, Ren Binbin, Wang Jingying. Major histocompatibility complex regulates immune responses in Parkinson’s disease[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5469-5477.
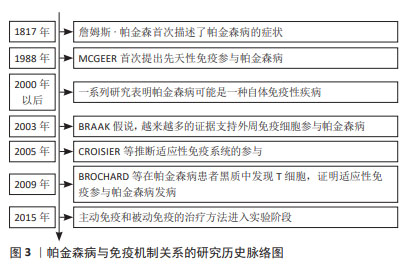
2.2 帕金森病中的免疫反应
2.2.1 先天性免疫 外周巨噬细胞能够对内源性刺激作出反应,发挥致病和保护功能,小胶质细胞也是如此。当暴露于内源性刺激时,小胶质细胞即被激活。活化的小胶质细胞释放出多种物质,包括促炎细胞因子、神经毒性蛋白、趋化因子、抗炎细胞因子和神经营养因子[10]。小胶质细胞有2种极性状态:M1表型与促炎和神经毒性反应相关,而M2表型主要介导抗炎和神经保护作用。M1小胶质细胞可以被错误折叠的蛋白质和环境毒素诱导,吞噬细胞,清除细胞碎片,以及释放促炎细胞因子:白细胞介素1β、白细胞介素6、白细胞介素12、白细胞介素17、白细胞介素18、白细胞介素23、肿瘤坏死因子α、干扰素γ和一氧化氮以及趋化因子CCL2[11]。M1小胶质细胞表型标记物包括诱导型一氧化氮合酶、环氧合酶2、MHC-Ⅱ和CD86等[12]。M2小胶质细胞的激活涉及多种机制,包括免疫调节、炎症抑制、修复和损伤消退,并且能产生一系列递质,如抗炎因子、细胞外基质蛋白、糖皮质激素和其他物质,见表1。
先天免疫参与帕金森病最早是由MCGEER等[5]提出的,他们发现帕金森病患者大脑显示出高水平的反应性小胶质细胞,并且在黑质和壳核中人类白细胞抗原D相关表达增加。GWAS表明人类白细胞抗原区域的变异与散发性帕金森病有关[17]。正电子发射断层扫描研究还发现,在不同的大脑区域,如脑干、基底节和额叶,小胶质细胞被广泛激活[6]。此外,在一些帕金森病小鼠模型中还观察到黑质和纹状体中的小胶质细胞增多[18]。不同脑区的反应性小胶质细胞伴随着较高水平的促炎细胞因子,如肿瘤坏死因子α、白细胞介素1β、白细胞介素6和干扰素γ,表明小胶质细胞参与了帕金森病发展过程中的神经炎症[19]。事实上,神经炎症被认为是一把“双刃剑”。活化的小胶质细胞具有神经保护性,有助于清除外来病原体和毒素。相反,慢性小胶质细胞增多症可导致帕金森病的细胞毒性和神经元丢失[20]。长期过度激活的小胶质细胞会加速细胞应激,影响记忆,并损害神经元的可塑性。随着帕金森病的进展,凋亡的神经细胞释放基质金属蛋白酶3、三磷酸腺苷和α-突触核蛋白,进一步激活小胶质细胞,导致多巴胺神经元变性。多项研究表明,帕金森病患者大脑中激活的小胶质细胞会加剧神经变性,而死亡多巴胺神经元释放的人类神经黑色素会引起趋化性并增加小胶质细胞培养物中的促炎物质[21-22]。BUTLER等[23]研究表明帕金森病患者中存在与M1激活相关的炎症标志物,例如MHC-Ⅱ、肿瘤坏死因子α和白细胞介素6。研究发现,帕金森病患者的脑脊液和纹状体样本中细胞因子水平升高,包括肿瘤坏死因子α、白细胞介素6和白细胞介素1β[24]。此外,通过免疫组化发现,在帕金森病患者黑质样本的阿米巴样小胶质细胞中,诱导型一氧化氮合酶、环氧合酶1和环氧合酶2的表达增加,同时黑质中细胞因子白细胞介素6、肿瘤坏死因子α水平升高[25],可见在小胶质细胞激活或增生时往往表现为M1促炎和M2抗炎两种不同的表型。促炎和抗炎的小胶质细胞可能在帕金森病脑中共存,小胶质细胞表型的复杂变化维持并加剧帕金森病的神经病理发展。小胶质细胞与多种神经退行性疾病有关,见表2。在阿尔茨海默病老年人脑中,已经报道了促炎和抗炎小胶质细胞表型的不平衡。因此,有理由推测帕金森病脑内存在一系列激活的小胶质细胞,它们聚集在一起,通过释放细胞因子来推动疾病的进展。
2.2.2 适应性免疫 外周T细胞由不同的亚群组成,当幼稚T细胞遇到树突状细胞呈递的抗原和共刺激配体时,引发免疫反应,调控白细胞介素2的产生,并增殖和分化为效应细胞,这些效应细胞迁移到不同的位点以促进病原体清除。一项关于死后帕金森病患者大脑的研究发现,在靠近血管和神经黑素阳性神经元的黑质中检测到了CD4+和CD8+ T细胞[30],这表明T细胞在帕金森病发病中具有重要作用。LAWTON等[31]通过观察帕金森病脑组织、血液和脑脊液发现,MHCⅡ+-APC之间的相互作用导致T细胞衍生的细胞因子表达升高,特别是干扰素γ和肿瘤坏死因子。SAUNDERS等[32]首次证明了人类帕金森病的运动障碍严重程度与T细胞的表型相关,研究还显示帕金森病中效应/记忆T细胞增加,CD4+CD45RA+静息/na?ve T细胞水平降低,这些改变与疾病进展有关。另一项研究表明在帕金森病小鼠中,CD4+和CD8+ T细胞的浸润程度明显升高[33],这一现象也可在帕金森病患者的大脑中发现。这些研究证明T细胞可能影响神经退行性变。因此,帕金森病进展与T细胞表型和功能有关这一观点被更多学者认可。调节适应性免疫应答可以影响帕金森病和其他神经退行性疾病的病理变化。
虽然多项研究显示B细胞能够通过外周作用引发中枢神经系统疾病,但迄今为止对B细胞在帕金森病中的研究还很有限[34]。B淋巴细胞是免疫系统的抗体分泌细胞,是体液免疫的关键递质,因为它们分泌的免疫球蛋白可以在远离实际分泌部位的组织或细胞产生作用[35]。鉴于帕金森病的慢性炎症性质,体液免疫可能在帕金森病的进展中发挥重要作用。在稳态条件下,B细胞少量存在于中枢神经系统薄壁组织(约0.1个细胞/cm2)和血管周围空间,并且这一B细胞亚群的数量和/或效应功能可以提高[36],这表明B细胞在免疫监视和抗原特异性记忆中发挥作用[34]。帕金森病患者的大脑中未检测到B细胞,但在黑质的多巴胺能神经元和中枢神经系统的路易小体上检测到IgG沉积[37]。已有研究证明帕金森病中自身抗体减少,B细胞通过清除细胞外病理性α-突触核蛋白的途径发挥神经保护作用[38]。α-突触核蛋白抗体水平在遗传性帕金森病、散发性帕金森病患者的血液和脑脊液中升高[39]。有学者对这些相互矛盾的结果进行了分析,认为队列、对照和技术方法的差异可能是造成结果不同的原因[40]。尽管目前尚不清楚这些衍生的α-突触核蛋白抗体是否具有神经保护作用,但体外实验表明,抗体有助于α-突触核蛋白清除[41],体内研究也支持这一观点。
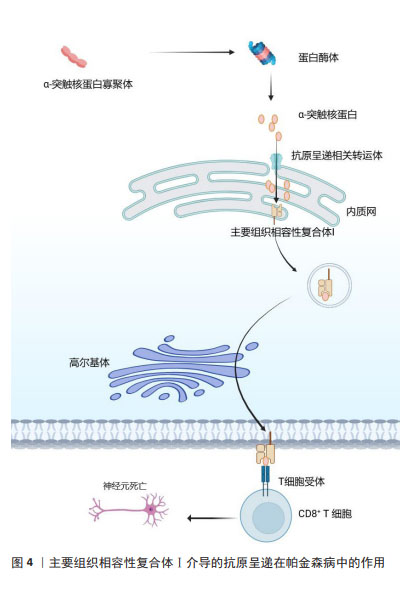
2.3 MHC在免疫中的作用 在人类中,MHC也称为人类白细胞抗原,MHC位点分为3类:Ⅰ类(人类白细胞抗原A、B、C)、Ⅱ类(人类白细胞抗原DR、DP、DQ)和Ⅲ类[42]。几乎所有有核细胞都表达MHC-Ⅰ,而MHC-Ⅱ分子仅位于APC表面,包括B细胞、树突状细胞、小胶质细胞、巨噬细胞和胸腺上皮细胞[43]。MHC-Ⅰ将源自细胞质和细胞核的细胞内蛋白片段呈递给细胞毒性CD8+ T细胞或NK细胞[44]。这些细胞内蛋白在细胞质中被蛋白酶体降解为寡肽,并通过与抗原呈递相关的转运蛋白转位到内质网中以接触MHC-Ⅰ。MHC-Ⅰ和肽在内质网中组装后,被转运至高尔基体并根据分泌途径到达细胞表面[45]。MHC-Ⅰ介导的抗原呈递在帕金森病中的作用见图4。胞外肽通过MHC-Ⅱ分子呈递给CD4+ T细胞。与MHC-Ⅰ分子类似,MHC-Ⅱ被组装并分别运输到内质网和高尔基体,然后肽装载过程将在MHC-Ⅱ类室(pH 5.5)或细胞表面(pH 7.2)完成[46]。在低pH值条件下,不变链是一种伴侣蛋白,可保护MHC-Ⅱ的肽结合裂口不与细胞内肽结合,并促进MHC-Ⅱ转移至MⅡC,然后不变链被降解为一种小肽,称为Ⅱ类相关不变链肽。人类白细胞抗原DM是一种MHC-Ⅱ类伴侣蛋白,可去除CLIP/MHC复合物并促进抗原肽与MHC-Ⅱ的结合[47]。MHC-Ⅱ介导的抗原呈递在帕金森病中的作用见图5。MHC-Ⅲ编码不同的产物,包括热休克蛋白、肿瘤坏死因子家族蛋白(肿瘤坏死因子α、肿瘤坏死因子β)和不同过程中的补体成分[48]。
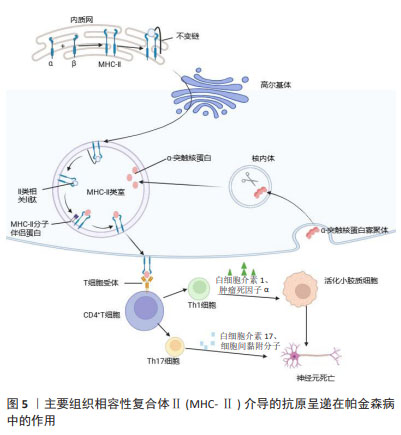
通常,活化的CD8+ T细胞会增殖并释放细胞因子和裂解分子(例如颗粒酶和穿孔素),从而促进受感染细胞的裂解。与之相反,CD4+ T细胞产生效应细胞因子,通过增强先天免疫系统和B细胞功能来“帮助”介导病原体清除[49]。B细胞上的MHC-Ⅱ有助于CD4+ T细胞对T细胞依赖性抗原的发育、分化和效应子功能作出应答[50]。在同源B细胞-T细胞相互作用期间,MHC-Ⅱ对于B细胞活化、增殖和分化具有关键作用[51]。与MHC-Ⅱ- B细胞相比,MHC-Ⅱ+B细胞在增殖、分化为浆母细胞或生殖中心B细胞和同种型转换方面具有显著优势[51]。
CHIRMULE等[52]认为在同源B-T细胞相互作用期间的B细胞活化信号主要由CD40介导。然而,T细胞依赖性IgM的产生可以在不存在CD40信号的情况下进行。研究表明,MHC-Ⅱ除了抗原呈递能力外,还促进T细胞依赖性IgM产生信号分子[50-53]。在人类和啮齿类动物中,MHC-Ⅱ是与α-突触核蛋白神经元病理学直接相关的免疫标志物[54]。在大脑中,小胶质细胞可以充当抗原呈递细胞,小胶质细胞摄取α-突触核蛋白后,在核内体中对其进行加工,并通过MHC-Ⅱ呈递给T细胞[54-55]。MHC-Ⅱ的差异表达调节α-突触核蛋白诱导的小胶质细胞激活和多巴胺能神经退行性变。因此,小胶质细胞活化程度、MHC-Ⅱ表达和α-突触核蛋白病理是相互依赖的[56]。JIMENEZ-FERRER等[57]研究证明Mhc2ta的等位基因变异与MHC-Ⅱ的低表达水平相关,进而导致与小胶质细胞高反应相关的大鼠α-突触核蛋白毒性增加。因此,适应性免疫信号(MHCⅡ-CD4 T细胞)异常将对α-突触核蛋白损伤的神经元存活产生影响。人类白细胞抗原区域中编码MHC-Ⅱ分子的常见变异与帕金森病风险相 关[58-59],并且MHC-Ⅱ+细胞存在于帕金森病脑中α-突触核蛋白附近[7],以及基于α-突触核蛋白过表达和纹状体注射预形成原纤维的帕金森病模型中[60]。此外,在帕金森病患者中发现循环CD4+ T淋巴细胞能够对MHC-Ⅱ呈递的α-突触核蛋白作出反应[61]。这些发现有力地支持了α-突触核蛋白病理与MHC-Ⅱ介导的抗原呈递之间的功能联系。HARMS等[62]也发现,病理性α-突触核蛋白的吞噬作用将导致蛋白质进入MHC系统,从而通过小胶质细胞(或其他细胞)将α-突触核蛋白肽呈递给适应性系统,这导致了适应性免疫系统的招募以及T细胞和B细胞的激活。CEBRIáN等[63]提出在少数儿茶酚胺神经元死亡的情况下,α-突触核蛋白和神经黑色素被释放到细胞外空间激活小胶质细胞,进而释放促炎物质,如干扰素γ,这将诱导儿茶酚胺神经元膜上MHC-Ⅰ的表达。MHC-Ⅰ将细胞间肽呈递在细胞表面以用于免疫监视,当CD8+ T细胞将肽识别为外源蛋白时,它们会攻击呈递细胞并诱导细胞凋亡[64]。WANG等[65]也发现,在1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的帕金森病模型小鼠中,多巴胺能神经元表现出强烈的MHC-Ⅰ阳性,并有明显的T细胞浸润。由细胞损伤触发的错误折叠蛋白的抗原呈递在细胞表面,这一现象激活了自身免疫反应并诱导T细胞的攻击,导致了多巴胺能神经元的死亡。因此,黑质中MHC-Ⅰ基因敲除能够降低CD8+ T细胞浸润程度,从而减少多巴胺能神经元的丢失。在帕金森病中,免疫系统处于激活状态并伴有炎症反应,这通常会加剧神经毒性和神经变性。值得注意的是,先天性免疫和适应性免疫依赖于MHC。因此,MHC的表达是免疫激活的重要标志。
2.4 MHC在帕金森病炎症反应中的表达 在中枢神经系统中,除APC外,神经胶质细胞也具有抗原呈递功能,并且MHC主要在小胶质细胞表达[66]。作为大脑中的常驻巨噬细胞和初级免疫细胞,小胶质细胞能够对感染和损伤作出反应[67]。有研究显示,在帕金森病患者的黑质和纹状体区域检测到了MHC-Ⅱ免疫反应性小胶质细胞,这些小胶质细胞位于少数剩余的黑质多巴胺神经元附近,表现出活化细胞和吞噬细胞的形态特征,类似于衰老细胞形态[68],并且人类白细胞抗原免疫反应性增加,一些小胶质细胞吞噬细胞化神经黑色素[69]。中脑的小胶质细胞类型不同于大脑其他区域,这表明小胶质细胞的区域差异可能调节帕金森病患者多巴胺能神经元的易感性。MAGNUSEN等[70]认为,在帕金森病的进展过程中小胶质细胞从“静息”状态到“激活”状态,激活的小胶质细胞与阿米巴样细胞(M1表型)相同,释放炎症细胞因子和趋化因子。脑、脑脊液和外周血中的这些细胞因子和趋化因子破坏血脑屏障,并将淋巴细胞吸引到神经元损伤部位加剧了神经炎症。肠道微生物群与小胶质细胞相互作用,调节小胶质细胞的成熟和功能,从而参与炎症反应[71]。研究表明,表达MHC-Ⅱ的肠上皮细胞可促进调节性T细胞在固有层和肠上皮中的扩张[72]。在干扰素γ存在的情况下,人脐静脉内皮细胞表达MHC-Ⅱ和人类白细胞抗原DM[73]。此外,人类白细胞抗原与肠道微生物群有关。在共享更多人类白细胞抗原等位基因的个体之间,微生物群相似性通常更高。ABELLANAS等[74]通过向小鼠腹腔注射脂多糖诱导帕金森病模型,观察到小胶质细胞被广泛激活,小鼠海马、中脑和纹状体中促炎细胞因子肿瘤坏死因子α、白细胞介素1β和白细胞介素6水平增加,但在皮质中只有肿瘤坏死因子α升高;此外,中脑小胶质细胞中MHC-Ⅱ的密度显著高于其他脑区。与盐水处理的小鼠相比,1-甲基-4-苯基-1,2,3,6-四氢吡啶处理后小鼠腹侧中脑多巴胺能神经元数量显著减少,MHC-Ⅱ β链mRNA增加,小胶质细胞激活,MHC-Ⅱ和Iba1的标志物位于同一位置。而MHC-Ⅱ的遗传消融可保护黑质多巴胺神经元免受1-甲基-4-苯基-1,2,3,6-四氢吡啶毒性的影响,并且MHC-Ⅱ缺失小鼠小胶质细胞减少[75]。1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的氧化应激也会增加多巴胺能神经元中MHC-Ⅰ的表达和CD8+ T细胞的间质浸润[64]。帕金森病患者血清和脑脊液中促炎因子和抗炎因子水平均升高,证明M1和M2小胶质细胞可能在脑中并存,小胶质细胞表型的复杂变化可能会加剧神经退行性变[76]。
通过体外结合实验,检测出呈递α-突触核蛋白的人类白细胞抗原等位基因,包括处于连锁不平衡状态的人类白细胞抗原Ⅱ类变体DRB1*15:01 和 DRB5*01:01[59]。一般而言,帕金森病患者的人类白细胞抗原分子表达较高,尤其是人类白细胞抗原Ⅱ类,这与其可能通过诱导炎症性环境来增强帕金森病易感性的研究结果一致[77]。星形胶质细胞发挥调节血脑屏障通透性和分泌神经营养因子的作用,促进多巴胺能神经元的存活和分化。因此,星形胶质细胞被认为是帕金森病进展的重要因素[78]。在帕金森病患者死后脑组织中,MHC-Ⅱ与星形细胞标志物共定位,并且星形胶质细胞周围有CD4+ T细胞浸润[79]。此外,在暴露于干扰素γ后,MHC-Ⅱ在星形胶质细胞中表达增加,效应性T细胞在体外也被诱导分化和增殖[80]。值得注意的是,星形胶质细胞通过分泌肿瘤坏死因子α、白细胞介素1α和C1q,在活化的小胶质细胞诱导下转变为神经毒性A1状态,进一步释放促炎因子,加剧炎性反应[81]。
为了验证T细胞在帕金森病中驱动炎症反应,许多研究报告了T细胞亚群的变化,其中最显著的是幼稚细胞、CD4 T辅助细胞(Th)、细胞毒性T细胞(CD8)和T调节细胞的减少,而其他研究报告总体数量没有变化或增加[82]。帕金森病患者的Th减少,与Th2、Th17和调节性T细胞数量的减少有关;此外,帕金森病患者的CD4- Th细胞表现出偏向Th1的免疫反应,干扰素γ和肿瘤坏死因子增加[83]。受损的神经元可以将α-突触核蛋白(修饰/错误折叠)释放到神经元外环境中,这可以通过微环境中的小胶质细胞激活引起神经炎症。SUBBARAYAN及其同事[84]的研究显示α-突触核蛋白表达会改变无胸腺裸鼠和杂合裸鼠的小胶质细胞,T细胞的存在会促进小胶质细胞表达Iba1以及黑质纹状体中MHC-Ⅱ的上调,表明T细胞的存在可能与小胶质细胞(单核细胞)中MHC-Ⅱ上调有关,这可能加速多巴胺能神经元丢失。此外,有研究表明干扰素γ上调多巴胺能神经元表面MHC-Ⅰ的表达,向CD8+ T细胞呈递抗原[85]。WANG等[65]的动物实验显示,在1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的帕金森病模型小鼠中,通过干扰黑质中的腺病毒沉默内源性MHC-Ⅰ可减少MHC-Ⅰ+多巴胺能神经元、多巴胺能神经元死亡和T细胞浸润。因此,MHC-Ⅰ使多巴胺能神经元更容易受到自身免疫的影响。
2.5 α-突触核蛋白引起的免疫和炎症反应 α-突触核蛋白的异常积聚是帕金森病的重要病理标志,也是先天免疫反应的发起者,可激活小胶质细胞和炎症反应。SULZER及其同事的一系列研究进一步支持α-突触核蛋白与免疫反应之间存在联系。在帕金森病神经炎症期间,小胶质细胞大量产生促炎细胞因子,免疫细胞被募集到中枢神经系统,导致血脑屏障通透性增加[86]。该小组的一篇论文表明,这一过程在帕金森病患者中引发了针对α-突触核蛋白表位的免疫反应,这些帕金森病患者具有特定的MHC等位基因。已经确定了两个抗原区:第一个靠近N端(称为Y39区),第二个靠近C端,即S129区,包含氨基酸残基S129,其磷酸化与α-突触核蛋白病理有关[61]。另一方面,人类白细胞抗原Ⅰ类等位基因A*11:01与2个人类白细胞抗原Ⅱ类变异存在轻度连锁不平衡,可在Y39区被较短的α-突触核蛋白肽高亲和力结合,帕金森病患者对α-突触核蛋白的免疫应答同时有人类白细胞抗原Ⅰ和人类白细胞抗原Ⅱ类成分[87]。因此,帕金森病中的炎症可能既起保护作用又起有害作用,这些发现支持这样一个事实,即帕金森病的免疫疗法可以通过提高免疫系统对α-突触核蛋白的耐受性或抑制免疫系统的过度活跃反应来改善[82]。
神经元分泌的α-突触核蛋白不仅可以在邻近细胞的胞浆内引起毒性,还可能在细胞外间隙引起毒性,这会激活胶质细胞并诱导慢性炎症(即帕金森病的常见病理特征),从而促进整个大脑的病理发展。KAM等[78]发现α-突触核蛋白还可以激活小胶质细胞中的核因子κB或结合小胶质细胞表面的Fcγ受体ⅡB,并降低小胶质细胞的吞噬作用。暴露于大鼠黑质纹状体中的α-突触核蛋白纤维可促进小胶质细胞的快速激活和MHC-Ⅱ的表达。在α-突触核蛋白呈递过程中,MHC显著表达。WILLIAMS等[88]经研究发现MHC-Ⅱ或MHC-Ⅱ反式激活剂CIITA的基因敲除可抑制α-突触核蛋白诱导的小胶质细胞激活、外周免疫细胞渗透、抗原呈递和多巴胺能神经元的退化。另一种MHC-Ⅱ反式激活剂Mhc2ta的等位基因变异可调节α-突触核蛋白过表达大鼠的MHC-Ⅱ表达、小胶质细胞激活、多巴胺能神经变性和运动缺陷[57]。
2.6 帕金森病的免疫治疗 α-突触核蛋白与帕金森病的免疫反应密切相关,成为治疗帕金森病的重要靶点。与α-突触核蛋白相关的治疗方法有2种:增加α-突触核蛋白的清除率,以及通过接种α-突触核蛋白疫苗提供神经保护。单磷酰脂质A是一种Toll样受体4选择性激动剂,已被证明可通过Toll样受体4诱导小胶质细胞摄取α-突触核蛋白,从而减少α突触核蛋白聚集并保护多巴胺能神经元,进而提高α-突触核蛋白的清除率[89]。α-突触核蛋白疫苗已进入研发阶段,α-突触核蛋白/葡萄糖相关蛋白94联合疫苗能够通过抑制小胶质细胞激活和帕金森病小鼠模型的神经炎症来重塑疾病免疫环境[90]。另外一种比单克隆抗体小得多的纳米抗体更容易穿过血脑屏障并浸润到细胞外空间,分为单链可变片段和单域纳米体两种类型[91]。
ZHA等[92]报道了一种构象特异性的单链可变片段W20,纯化的W20可以结合到由不同淀粉样蛋白组装的各种低聚结构的共同表位上。注射纯化的泛淀粉样蛋白低聚物特异性单链可变片段可减轻帕金森病模型小鼠的运动(旷场、转棒试验)和认知(Morris水迷宫、物体识别试验)障碍。同时W20也降低了α-突触核蛋白的总体水平,维持纹状体中正常的酪氨酸氢化酶和多巴胺水平。这些研究表明靶向α-突触核蛋白的免疫疗法可能是治疗帕金森病的有效策略。另外,WILLIAMS及其同事[88]的研究显示,敲除或沉默CIITA基因能够降低α-突触核蛋白介导的MHC-Ⅱ在小胶质细胞上的表达,减少T细胞和单核细胞的浸润,以及缓解神经变性。通过靶向CIITA来干扰或调节抗原的处理和呈递是一种有前途的方法。目前免疫疗法在帕金森病临床试验中显示出可观的结果,但是临床功能恢复尚未得到证实。因此,制定一个可靠的方案来评估临床试验的有效性是十分必要的,这需要更加深入地研究探讨帕金森病的发生和发展。
| [1] BI R, FANG Z, YOU M, et al. Microglia Phenotype and Intracerebral Hemorrhage: A Balance of Yin and Yang. Front Cell Neurosci. 2021; 15:765205. [2] CHU Y, HIRST WD, FEDEROFF HJ, et al. Nigrostriatal tau pathology in parkinsonism and Parkinson’s disease. Brain. 2024;147(2):444-457. [3] SONG Z, LI W, HAN Y, et al. Association of immune cell traits with Parkinson’s disease: a Mendelian randomization study. Front Aging Neurosci. 2024;16:1340110. [4] YANG Q, LV Z, WANG M, et al. LATS1/2 loss promote tumor immune evasion in endometrial cancer through downregulating MHC-I expression. J Exp Clin Cancer Res. 2024;43(1):54. [5] MCGEER PL, ITAGAKI S, BOYES BE, et al. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285-1291. [6] HEIDARI A, YAZDANPANAH N, REZAEI N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J Neuroinflammation. 2022;19(1):135. [7] CROISIER E, MORAN LB, DEXTER DT, et al. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. [8] BROCHARD V, COMBADIÈRE B, PRIGENT A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119(1):182-192. [9] JANKOVIC J, GOODMAN I, SAFIRSTEIN B, et al. Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-α-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2018;75(10):1206-1214. [10] SEVENICH L. Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer. Front Immunol. 2018;9:697. [11] XUE Y, NIE D, WANG LJ, et al. Microglial Polarization: Novel Therapeutic Strategy against Ischemic Stroke. Aging Dis. 2021;12(2): 466-479. [12] WENDIMU MY, HOOKS SB. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells. 2022;11(13):2091. [13] GUO S, WANG H, YIN Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front Aging Neurosci. 2022;14: 815347. [14] LUKACOVA N, KISUCKA A, KISS BIMBOVA K, et al. Glial-Neuronal Interactions in Pathogenesis and Treatment of Spinal Cord Injury. Int J Mol Sci. 2021;22(24):13577. [15] WEI Y, LI X. Different phenotypes of microglia in animal models of Alzheimer disease. Immun Ageing. 2022;19(1):44. [16] KISUCKÁ A, BIMBOVÁ K, BAČOVÁ M, et al. Activation of Neuroprotective Microglia and Astrocytes at the Lesion Site and in the Adjacent Segments Is Crucial for Spontaneous Locomotor Recovery after Spinal Cord Injury. Cells. 2021;10(8):1943. [17] HILL-BURNS EM, FACTOR SA, ZABETIAN CP, et al. Evidence for more than one Parkinson’s disease-associated variant within the HLA region. PLoS One. 2011;6(11):e27109. [18] ISIK S, YEMAN KIYAK B, AKBAYIR R, et al. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells. 2023;12(7):1012. [19] ZHU R, LUO Y, LI S, et al. The role of microglial autophagy in Parkinson’s disease. Front Aging Neurosci. 2022;14:1039780. [20] LECOURS C, BORDELEAU M, CANTIN L, et al. Microglial Implication in Parkinson’s Disease: Loss of Beneficial Physiological Roles or Gain of Inflammatory Functions? Front Cell Neurosci. 2018;12:282. [21] SANDHU JK, KULKA M. Decoding Mast Cell-Microglia Communication in Neurodegenerative Diseases. Int J Mol Sci. 2021;22(3):1093. [22] WILMS H, ZECCA L, ROSENSTIEL P, et al. Inflammation in Parkinson’s diseases and other neurodegenerative diseases: cause and therapeutic implications. Curr Pharm Des. 2007;13(18):1925-1928. [23] BUTLER CA, POPESCU AS, KITCHENER EJA, et al. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem. 2021;158(3):621-639. [24] LANZA M, CASILI G, CAMPOLO M, et al. Immunomodulatory Effect of Microglia-Released Cytokines in Gliomas. Brain Sci. 2021;11(4):466. [25] KNOTT C, STERN G, WILKIN GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16(6):724-739. [26] BASURCO L, ABELLANAS MA, AYERRA L, et al. Microglia and astrocyte activation is region-dependent in the α-synuclein mouse model of Parkinson’s disease. Glia. 2023;71(3):571-587. [27] IMAMURA K, HISHIKAWA N, SAWADA M, et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106(6): 518-526. [28] WILLIAMS-GRAY CH, WIJEYEKOON R, YARNALL AJ, et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord. 2016;31(7):995-1003. [29] LIU WW, WEI SZ, HUANG GD, et al. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson’s disease mouse model. FASEB J. 2020;34(5):6570-6581. [30] SOMMER A, MARXREITER F, KRACH F, et al. Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease. Cell Stem Cell. 2018;23(1):123-131.e6. [31] LAWTON M, BAIG F, TOULSON G, et al. Blood biomarkers with Parkinson’s disease clusters and prognosis: The oxford discovery cohort. Mov Disord. 2020;35(2):279-287. [32] SAUNDERS JA, ESTES KA, KOSLOSKI LM, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7(4):927-938. [33] SAPONJIC J, MEJÍAS R, NIKOLOVSKI N, et al. Experimental Models to Study Immune Dysfunction in the Pathogenesis of Parkinson’s Disease. Int J Mol Sci. 2024;25(8):4330. [34] SABATINO JJ JR, PRÖBSTEL AK, ZAMVIL SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20(12):728-745. [35] NAKAGAWA R, CALADO DP. Positive Selection in the Light Zone of Germinal Centers. Front Immunol. 2021;12:661678. [36] MACHADO-SANTOS J, SAJI E, TRÖSCHER AR, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141(7):2066-2082. [37] ORR CF, ROWE DB, MIZUNO Y, et al. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain. 2005; 128(Pt 11):2665-2674. [38] BRUDEK T, WINGE K, FOLKE J, et al. Autoimmune antibody decline in Parkinson’s disease and Multiple System Atrophy; a step towards immunotherapeutic strategies. Mol Neurodegener. 2017;12(1):44. [39] AKHTAR RS, LICATA JP, LUK KC, et al. Measurements of auto-antibodies to α-synuclein in the serum and cerebral spinal fluids of patients with Parkinson’s disease. J Neurochem. 2018;145(6):489-503. [40] SCOTT KM, KOULI A, YEOH SL, et al. A Systematic Review and Meta-Analysis of Alpha Synuclein Auto-Antibodies in Parkinson’s Disease. Front Neurol. 2018;9:815. [41] BAE EJ, LEE HJ, ROCKENSTEIN E, et al. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):13454-13469. [42] ROSSJOHN J, GRAS S, MILES JJ, et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol. 2015;33:169-200. [43] ROCHE PA, FURUTA K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15(4): 203-216. [44] KAUFMAN J. Unfinished Business: Evolution of the MHC and the Adaptive Immune System of Jawed Vertebrates. Annu Rev Immunol. 2018;36:383-409. [45] ZAITOUA AJ, KAUR A, RAGHAVAN M. Variations in MHC class I antigen presentation and immunopeptidome selection pathways. F1000Res. 2020;9:F1000 Faculty Rev-1177. [46] MÜNZ C. The Macroautophagy Machinery in MHC Restricted Antigen Presentation. Front Immunol. 2021;12:628429. [47] WIECZOREK M, ABUALROUS ET, STICHT J, et al. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front Immunol. 2017;8:292. [48] SZNARKOWSKA A, MIKAC S, PILCH M. MHC Class I Regulation: The Origin Perspective. Cancers (Basel). 2020;12(5):1155. [49] SCHONHOFF AM, WILLIAMS GP, WALLEN ZD, et al. Innate and adaptive immune responses in Parkinson’s disease. Prog Brain Res. 2020;252: 169-216. [50] KATIKANENI DS, JIN L. B cell MHC class II signaling: A story of life and death. Hum Immunol. 2019;80(1):37-43. [51] ROLAND MM, PEACOCK TE, HALL N, et al. B-cell-specific MhcII regulates microbiota composition in a primarily IgA-independent manner. Front Immunol. 2023;14:1253674. [52] CHIRMULE N, TAZELAAR J, WILSON JM. Th2-dependent B cell responses in the absence of CD40-CD40 ligand interactions. J Immunol. 2000;164(1):248-255. [53] JORDAN MB, MILLS DM, KAPPLER J, et al. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304(5678):1808-1810. [54] FERREIRA SA, ROMERO-RAMOS M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front Cell Neurosci. 2018;12:247. [55] ALMOLDA B, GONZÁLEZ B, CASTELLANO B. Are Microglial Cells the Regulators of Lymphocyte Responses in the CNS? Front Cell Neurosci. 2015;9:440. [56] JIMENEZ-FERRER I, JEWETT M, TONTANAHAL A, et al. Allelic difference in Mhc2ta confers altered microglial activation and susceptibility to α-synuclein-induced dopaminergic neurodegeneration. Neurobiol Dis. 2017;106:279-290. [57] JIMENEZ-FERRER I, BÄCKSTRÖM F, DUEÑAS-REY A, et al. The MHC class II transactivator modulates seeded alpha-synuclein pathology and dopaminergic neurodegeneration in an in vivo rat model of Parkinson’s disease. Brain Behav Immun. 2021;91:369-382. [58] GOPINATH A, MACKIE PM, PHAN LT, et al. The complex role of inflammation and gliotransmitters in Parkinson’s disease. Neurobiol Dis. 2023;176:105940. [59] KANNARKAT GT, COOK DA, LEE JK, et al. Common Genetic Variant Association with Altered HLA Expression, Synergy with Pyrethroid Exposure, and Risk for Parkinson’s Disease: An Observational and Case-Control Study. NPJ Parkinsons Dis. 2015;1:15002. [60] DUFFY MF, COLLIER TJ, PATTERSON JR, et al. Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. J Neuroinflammation. 2018;15(1):129. [61] SULZER D, ALCALAY RN, GARRETTI F, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017; 546(7660):656-661. [62] HARMS AS, CAO S, ROWSE AL, et al. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33(23):9592-9600. [63] CEBRIÁN C, LOIKE JD, SULZER D. Neuronal MHC-I expression and its implications in synaptic function, axonal regeneration and Parkinson’s and other brain diseases. Front Neuroanat. 2014;8:114. [64] HOBSON BD, SULZER D. Neuronal Presentation of Antigen and Its Possible Role in Parkinson’s Disease. J Parkinsons Dis. 2022;12(s1): S137-S147. [65] WANG BY, YE YY, QIAN C, et al. Stress increases MHC-I expression in dopaminergic neurons and induces autoimmune activation in Parkinson’s disease. Neural Regen Res. 2021;16(12):2521-2527. [66] ALISEYCHIK MP, ANDREEVA TV, ROGAEV EI. Immunogenetic Factors of Neurodegenerative Diseases: The Role of HLA Class II. Biochemistry (Mosc). 2018;83(9):1104-1116. [67] XU S, LU J, SHAO A, et al. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front Immunol. 2020;11:294. [68] HARLEY J, SANTOSA MM, NG CY, et al. Telomere shortening induces aging-associated phenotypes in hiPSC-derived neurons and astrocytes. Biogerontology. 2024;25(2):341-360. [69] QIAO C, NIU G, ZHAO W, et al. RIPK1-Induced A1 Reactive Astrocytes in Brain in MPTP-Treated Murine Model of Parkinson’s Disease. Brain Sci. 2023;13(5):733. [70] MAGNUSEN AF, HATTON SL, RANI R, et al. Genetic Defects and Pro-inflammatory Cytokines in Parkinson’s Disease. Front Neurol. 2021;12:636139. [71] DENG W, YI P, XIONG Y, et al. Gut Metabolites Acting on the Gut-Brain Axis: Regulating the Functional State of Microglia. Aging Dis. 2024; 15(2):480-502. [72] HEUBERGER C, POTT J, MALOY KJ. Why do intestinal epithelial cells express MHC class II? Immunology. 2021;162(4):357-367. [73] WOSEN JE, ILSTAD-MINNIHAN A, CO JY, et al. Human Intestinal Enteroids Model MHC-II in the Gut Epithelium. Front Immunol. 2019; 10:1970. [74] ABELLANAS MA, ZAMARBIDE M, BASURCO L, et al. Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions. J Neuroinflammation. 2019;16(1):233. [75] MARTIN HL, SANTORO M, MUSTAFA S, et al. Evidence for a role of adaptive immune response in the disease pathogenesis of the MPTP mouse model of Parkinson’s disease. Glia. 2016;64(3):386-395. [76] LIU TW, CHEN CM, CHANG KH. Biomarker of Neuroinflammation in Parkinson’s Disease. Int J Mol Sci. 2022;23(8):4148. [77] YU E, AMBATI A, ANDERSEN MS, et al. Fine mapping of the HLA locus in Parkinson’s disease in Europeans. NPJ Parkinsons Dis. 2021;7(1):84. [78] KAM TI, HINKLE JT, DAWSON TM, et al. Microglia and astrocyte dysfunction in parkinson’s disease. Neurobiol Dis. 2020;144:105028. [79] ROSTAMI J, FOTAKI G, SIROIS J, et al. Astrocytes have the capacity to act as antigen-presenting cells in the Parkinson’s disease brain. J Neuroinflammation. 2020;17(1):119. [80] WAISMAN A, JOHANN L. Antigen-presenting cell diversity for T cell reactivation in central nervous system autoimmunity. J Mol Med (Berl). 2018;96(12):1279-1292. [81] MASON HD, MCGAVERN DB. How the immune system shapes neurodegenerative diseases. Trends Neurosci. 2022;45(10):733-748. [82] GARRETTI F, AGALLIU D, LINDESTAM ARLEHAMN CS, et al. Autoimmunity in Parkinson’s Disease: The Role of α-Synuclein-Specific T Cells. Front Immunol. 2019;10:303. [83] KUSTRIMOVIC N, COMI C, MAGISTRELLI L, et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J Neuroinflammation. 2018;15(1):205. [84] SUBBARAYAN MS, HUDSON C, MOSS LD, et al. T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an α-synuclein rat model of Parkinson’s disease. J Neuroinflammation. 2020;17(1):242. [85] HARMS AS, FERREIRA SA, ROMERO-RAMOS M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol. 2021;141(4):527-545. [86] BADANJAK K, FIXEMER S, SMAJIĆ S, et al. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int J Mol Sci. 2021; 22(9):4676. [87] ZHU B, YIN D, ZHAO H, et al. The immunology of Parkinson’s disease. Semin Immunopathol. 2022;44(5):659-672. [88] WILLIAMS GP, SCHONHOFF AM, JURKUVENAITE A, et al. Targeting of the class II transactivator attenuates inflammation and neurodegeneration in an alpha-synuclein model of Parkinson’s disease. J Neuroinflammation. 2018;15(1):244. [89] VENEZIA S, REFOLO V, POLISSIDIS A, et al. Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy. Mol Neurodegener. 2017;12(1):52. [90] VILLADIEGO J, LABRADOR-GARRIDO A, FRANCO JM, et al. Immunization with α-synuclein/Grp94 reshapes peripheral immunity and suppresses microgliosis in a chronic Parkinsonism model. Glia. 2018;66(1): 191-205. [91] CASTONGUAY AM, GRAVEL C, LÉVESQUE M. Treating Parkinson’s Disease with Antibodies: Previous Studies and Future Directions. J Parkinsons Dis. 2021;11(1):71-92. [92] ZHA J, LIU XM, ZHU J, et al. A scFv antibody targeting common oligomeric epitope has potential for treating several amyloidoses. Sci Rep. 2016;6:36631. |
| [1] | 张艺博, 卢健棋, 毛美玲, 庞 延, 董 礼, 杨尚冰, 肖 湘. 类风湿关节炎与冠状动脉粥样硬化的因果关系:GWAS数据库血清代谢物和炎症因子数据[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 余 帅, 刘家伟, 朱 彬, 潘 檀, 李兴龙, 孙广峰, 于海洋, 丁 亚, 王宏亮. 小分子药物治疗骨关节炎的热点问题及应用前景[J]. 中国组织工程研究, 2025, 29(9): 1913-1922. |
| [3] | 王文涛, 侯振扬, 王熠军, 徐耀增. Apelin-13抑制巨噬细胞M1极化缓解全身炎症性骨丢失[J]. 中国组织工程研究, 2025, 29(8): 1548-1555. |
| [4] | 陈 帅, 金 杰, 韩化伟, 田宁晟, 李志伟. 两样本孟德尔随机化分析循环炎症细胞因子与骨密度的因果关联[J]. 中国组织工程研究, 2025, 29(8): 1556-1564. |
| [5] | 孙家琪, 卞 璐, 史文涛, 吴学潮, 鲁晓杰. 压电型机械门控离子通道组件1在大鼠压力性损伤中的作用机制[J]. 中国组织工程研究, 2025, 29(8): 1578-1584. |
| [6] | 李开颖, 魏晓歌, 宋 斐, 杨 楠, 赵振宁, 王 燕, 穆 静, 马惠昇. 理筋手法调控兔骨骼肌损伤修复中瘢痕形成的作用机制[J]. 中国组织工程研究, 2025, 29(8): 1600-1608. |
| [7] | 艾克帕尔·艾尔肯, 陈晓涛, 吾凡别克·巴合提. 成骨诱导人牙周膜干细胞来源外泌体促进炎症微环境下人牙周膜干细胞成骨分化[J]. 中国组织工程研究, 2025, 29(7): 1388-1394. |
| [8] | 何龙才, 宋文学, 明 江, 陈光唐, 王军浩, 廖益东, 崔君拴, 徐卡娅. SD大鼠乳鼠原代皮质神经元和小胶质细胞同时提取并培养的实验方法[J]. 中国组织工程研究, 2025, 29(7): 1395-1400. |
| [9] | 迟文鑫, 张存鑫, 高 凯, 吕超亮, 张科峰. 川陈皮素抑制BV2小胶质细胞炎症反应的机制[J]. 中国组织工程研究, 2025, 29(7): 1321-1327. |
| [10] | 喻 婷, 吕冬梅, 邓 浩, 孙 涛, 程 钎. 淫羊藿苷预处理增强人牙周膜干细胞对M1型巨噬细胞的影响[J]. 中国组织工程研究, 2025, 29(7): 1328-1335. |
| [11] | 赵瑞华, 陈思娴, 郭 杨, 石 磊, 吴承杰, 吴 毛, 杨光露, 张昊恒, 马 勇. 温肾通督方促进小鼠脊髓损伤的修复[J]. 中国组织工程研究, 2025, 29(6): 1118-1126. |
| [12] | 何 波, 陈 文, 马岁录, 何志军, 宋 渊, 李金鹏, 刘 涛, 魏晓涛, 王威威, 谢 婧. 皮瓣缺血再灌注损伤的发病机制及治疗进展[J]. 中国组织工程研究, 2025, 29(6): 1230-1238. |
| [13] | 白 静, 张 雪, 任 燕, 李月辉, 田晓宇. lncRNA-TNFRSF13C调控miR-1246对牙周细胞低氧诱导因子1α的作用[J]. 中国组织工程研究, 2025, 29(5): 928-935. |
| [14] | 支 芳, 朱满华, 熊 伟, 林星镇. 腰椎间盘突出症模型大鼠疼痛的针刺干预[J]. 中国组织工程研究, 2025, 29(5): 936-941. |
| [15] | 王荣荣, 黄玉珊, 李湘淼, 白金柱. 创伤性脊髓损伤急性期前列腺素E1对血管相关因子的调节和微循环功能的保护[J]. 中国组织工程研究, 2025, 29(5): 958-967. |
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者在2024年3月进行检索。
1.1.2 检索文献时限 自数据库建库起至2024年3月。
1.1.3 检索数据库 PubMed数据库。
1.1.4 检索词 检索词为“Parkinson’s disease,the major histocompatibility complex,innate immunity,adaptive immunity,microglia,T cell,B cell,α-syn,inflammation,MHC-Ⅰ,MHC-Ⅱ,immunotherapy”等。
1.1.5 检索文献类型 研究原著、综述、临床试验。
1.1.6 检索策略 PubMed数据库检索策略见图1。
1.1.7 检索文献量 初步检索文献量1 963篇。
1.2 入组标准1.2.1 纳入标准 ①帕金森病和免疫相关的研究;②帕金森病和MHC相关的研究;③在帕金森病中MHC调控免疫反应的相关研究;④α-突触核蛋白和MHC相关的研究。
1.2.2 排除标准 ①与研究内容不相关的文献;②质量较低的文献;③重复发表的文献。
1.2.3 文献质量评估 通过阅读文献的标题、摘要、关键词进行筛选,排除与研究目的相关性低、质量不高及重复文献,查阅全文,筛选出符合纳入标准的文献92篇,见图2。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
3.2 作者综述区别于他人他篇的特点 以往综述对免疫反应发生机制进行了详细阐述,探讨了免疫反应在帕金森病病理发生发展过程中的作用,并未深入总结探讨某一调节因子在帕金森病免疫中的作用。该文章在此基础上对MHC进行研究,从MHC介导免疫细胞的增殖、分化及分泌方面,探讨MHC如何通过调控免疫反应,缓解神经炎症,从而减缓疾病进程,为帕金森病的治疗提供新的
靶点。
3.3 综述的局限性 近年来,关于MHC在免疫疾病中的作用逐渐被人们所熟知。梳理大量文献发现,免疫反应在帕金森病病理进程中的具体作用机制尚未完全阐明,MHC调控免疫反应的机制有待深入研究。
3.4 综述的重要意义 随着社会老龄化,帕金森病已经成为影响老年人健康的主要疾病之一,严重影响患者生活质量,增加患者家庭和社会的负担。文章总结探讨MHC通过调控免疫和炎症在帕金森病中的作用,以及MHC对α-突触核蛋白的调控作用,为帕金森病的干预与治疗提供理论依据和潜在靶点。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
帕金森病:作为第二大神经退行性疾病,帕金森病临床表现为多个方面,最主要的是运动症状,如肌强直、运动迟缓、静止性震颤、姿势平衡障碍。除此之外,自主神经功能障碍和精神障碍(便秘、泌尿障碍、睡眠障碍、抑郁、焦虑、认知减退等)也对患者的生活产生严重影响。帕金森病的发病机制尚未完全阐明,多巴胺神经元缺失和α-突触核蛋白的聚集是其主要病理特征。#br# 主要组织相容性复合体:人类主要组织相容性复合体也称为人类白细胞抗原系统,分为Ⅰ、Ⅱ和Ⅲ类,它们可以结合来自细胞外或者细胞内的抗原肽,呈递给T细胞,在先天性免疫和适应性免疫反应中发挥不可或缺的作用。#br##br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
目前,专家学者们正在不断探索帕金森病的有效治疗方法。随着免疫机制在疾病中的作用被发掘,帕金森病的免疫治疗逐渐走入大众视野,成为治疗帕金森病的新靶点。基于α-突触核蛋白在帕金森病发生和发展中的重要作用,专家学者把视线放在了针对α-突触核蛋白的免疫疗法。多种抗体已进入研发阶段,包括完全性抗体和纳米抗体。这些抗体在实验中表现出可观的疗效,但是其在临床功能恢复方面的疗效尚未得到证实,并且缺乏评估临床试验疗效的策略。更好地了解α-突触核蛋白的解离、聚集和相关毒性及其细胞间传播机制,将有助于开发有效的抗体。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||