中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (14): 3034-3042.doi: 10.12307/2025.398
• 组织构建综述 tissue construction review • 上一篇 下一篇
肠道菌群、运动干预与呼吸系统疾病
殷 月1,冷思逸1,靳 攀2,陈子扬1,3,蒲 锐1,3
- 长江大学,1教育与体育学院,2医学部,3运动人体科学实验室,湖北省荆州市 434023
-
收稿日期:2024-06-03接受日期:2024-07-03出版日期:2025-05-18发布日期:2024-09-29 -
通讯作者:蒲锐,讲师,硕士研究生导师,长江大学,教育与体育学院,运动人体科学实验室,湖北省荆州市 434023 共同通讯作者:靳攀,副教授,硕士研究生导师,长江大学医学部,湖北省荆州市 434023 -
作者简介:殷月,女,2000年生,湖北省宜昌市人,汉族,在读硕士,主要从事运动健康促进研究。 -
基金资助:国家自然科学基金项目(81860386),项目负责人:靳攀;2022荆州市医疗卫生科技计划项目(2022HC36),项目负责人:靳攀
Intestinal flora, exercise intervention and respiratory diseases
Yin Yue1, Leng Siyi1, Jin Pan2, Chen Ziyang1, 3, Pu Rui1, 3
- 1College of Education and Sports Sciences, 2Health Science Center, 3Human Science Laboratory of Exercise, Yangtze University, Jingzhou 434023, Hubei Province, China
-
Received:2024-06-03Accepted:2024-07-03Online:2025-05-18Published:2024-09-29 -
Contact:Pu Rui, Lecturer, Master’s supervisor, College of Education and Sports Sciences, Yangtze University, Jingzhou 434023, Hubei Province, China; Human Science Laboratory of Exercise, Yangtze University, Jingzhou 434023, Hubei Province, China Co-corresponding author: Jin Pan, Associate professor, Master’s supervisor, Health Science Center, Yangtze University, Jingzhou 434023, Hubei Province, China -
About author:Yin Yue, Master candidate, College of Education and Sports Sciences, Yangtze University, Jingzhou 434023, Hubei Province, China -
Supported by:National Natural Science Foundation of China, No. 81860386 (to JP); 2022 Jingzhou Healthcare Science and Technology Program, No. 2022HC36 (to JP)
摘要:
文题释义:
肠道菌群:是人体内最大的微生物群落之一,与宿主之间形成了复杂的相互作用关系。肠道菌群不仅在肠胃道消化、机体代谢和免疫炎症等生理功能中发挥显著作用,还参与调控多种疾病的发生和发展。
“肠-肺轴”:是指肠道和肺部通过共生微生物而对免疫功能产生远距离交互影响,是一种双向轴,即呼吸道菌群、肠道菌群和呼吸系统疾病、消化系统疾病之间相互串扰。
背景:肠道菌群是位于人体胃肠道中的一种多样化和动态的微生物群落总称,对维持人体免疫和健康有着至关重要的作用。近年提出“肠-肺轴”概念,提示肠道菌群与肺密切相关,且运动可通过维持肠道菌群平衡调节呼吸系统疾病。
目的:综述了肠道菌群与肺炎、肺癌、哮喘病和慢性肺阻塞疾病等不同呼吸系统疾病的关系、不同运动方式对肠道菌群和呼吸系统疾病的影响,为深入探讨运动调控肠道菌群在呼吸系统疾病中的作用机制提供新的思路。
方法:检索1944-2024年间CNKI和PubMed数据库相关文献,中文检索词包括“肠道菌群、肠道细菌、呼吸系统疾病、肺炎、肺癌、哮喘病和慢性肺阻塞疾病、有氧运动、抗阻运动”等;英文检索词包括“Intestinal flora,Gut bacteria,Respiratory illness,Pneumonia,Lung cancer,Asthma,Chronic obstructive pulmonary diseases,Aerobic exercise、Resistance training”等,根据纳入和排除标准选择101篇文献进行归纳总结。
结果与结论:①肠道菌群在肺炎、肺癌、哮喘病和慢性肺阻塞疾病等多种呼吸系统疾病中发挥着重要的调节作用。②不同运动方式与肠道菌群密切相关:有氧运动可通过改善胰岛素敏感性、增加菌群多样性和抑制全身慢性炎症在肠道菌群的调控中发挥有益效应;抗阻运动可降低肠黏膜通透性并促进短链脂肪酸的产生;有氧联合抗阻运动也可提升肠道菌群多样性、影响肠道菌群组成。③运动可通过调节炎症反应和减轻氧化应激损伤,提高心肺功能和运动表现进而改善呼吸系统疾病。④运动通过调控肠道菌群抑制炎症反应、调节氧化应激、改善肠屏障通透性和维持肠道菌群稳态,在防治呼吸系统疾病中发挥关键作用。
https://orcid.org/0009-0003-4744-7053(殷月)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
殷 月, 冷思逸, 靳 攀, 陈子扬, 蒲 锐. 肠道菌群、运动干预与呼吸系统疾病[J]. 中国组织工程研究, 2025, 29(14): 3034-3042.
Yin Yue, Leng Siyi, Jin Pan, Chen Ziyang, Pu Rui . Intestinal flora, exercise intervention and respiratory diseases[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 3034-3042.
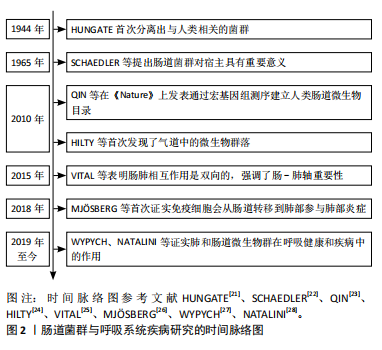
2.2 肠道菌群与呼吸系统疾病 大量研究结果表明,肠道菌群失衡与后期的疾病风险(如心血管疾病、癌症和呼吸系统疾病等)存在密切关联[29]。因此,文章对肠道菌群与各类呼吸系统疾病的关系进行深入分析。
2.2.1 肠道菌群与肺炎 肺炎是一种由病毒、细菌和真菌等所致的呼吸系统传染病,其发病率较高,易引发多种并发症。研究发现,大鼠肺疾病与肠道菌群失衡密切相关,主要特征为有益菌数量减少和致病菌数量增多[5,30]。另有研究表明,肺炎患者体内变形菌和不动杆菌与健康人群相比丰度较高[31]。同时,棒状杆菌属丰度的降低和链球菌属、奈瑟菌属、普雷沃氏菌属以及梭杆菌属丰度增加会引发呼吸道感染导致患肺炎风险增加[32]。此外,肠道菌群的多样性和菌群数量决定了肠道微生物生态平衡,肺炎患儿较健康儿童体内血清炎症因子与瘤胃球菌、丁酸梭菌的数量显著减少进而加剧炎症反应[33]。由此可见,肠道菌群丰度和多样性下降以及肠道菌群失调与肺炎的发生发展密切相关,提示肠道菌群可作为肺炎诊断的新型生物标记物。
肠道微生物在肺部感染中的研究主要以细菌和病毒感染为主,宿主肠道菌群的变化和失衡会伴有黏膜屏障和免疫功能受损从而引发肺炎[5]。研究发现,将肺炎球菌定植于肠道微生物组中,肺炎小鼠肠道微生物的多样性改变,拟杆菌科、普雷沃菌科和丹毒丝菌科的丰度显著降低,而嗜黏蛋白阿克曼氏菌和葡萄球菌科的丰度显著增加,提示肺炎球菌会影响上呼吸道和肠道菌群的分类和功能特征[34]。钱文娟[35]发现“银翘”可上调小鼠体内嗜胆菌属、真杆菌属和瘤胃球菌属丰度,改善肠道炎症及肠道屏障损伤促进机体平衡,进而改善肺炎。
上述研究提示,肺炎的发生发展与肠道菌群失调有关。调节菌群多样性、提高肠黏膜屏障功能和维持机体微生态平衡能影响肺炎的病理进程,但关于肠道菌群对肺炎具体生物学机制的相关研究较少,亟待进一步深入分析。
2.2.2 肠道菌群与肺癌 肺癌是全球范围内最常见且发病率和死亡率较高的恶性肿瘤,肠道菌群可调节机体代谢和免疫状态来影响肺癌的发生。研究表明,肺癌患者机体内厚壁菌门和变形菌门丰度较低,而拟杆菌门和梭杆菌门的丰度相对较高,提示肠道菌群与肺癌之间具有潜在联系[36]。有研究发现,菌群稳态失衡会诱发机体慢性炎症,降低抗原免疫应答和清除老化细胞的能力,加剧炎症状态并构成致癌环境,进而导致肺癌的发生和发展[37]。此外,肺癌患者与健康人群相比,肠内益生菌(如双歧杆菌、乳酸杆菌和瘤胃球菌)数量减少,致病菌肺炎克雷伯菌数量增加[38]。ZHANG等[39]采用的16SrRNA测定结果表明,肺癌患者肠道内放线菌和链球菌等致病菌含量显著增多,而具有抗肿瘤作用的普雷沃菌属丰度显著降低,表明致病菌会刺激肺癌病变。由此可见,肠道菌群可作为肺癌诊断的潜在标志物。
“肠-肺轴”会使肠道菌群失衡激活肠道免疫上调炎症因子表达,并通过血液循环到达肺部,引起中性粒细胞和巨噬细胞为代表的炎症细胞聚集,进而启动肺部的炎症级联反应[40]。同时,肠道菌群还可以通过“肠-肺轴”调节肠道和肺部细胞因子、化学因子和代谢产物的分泌,进而抑制肺癌的发生发展[41-43]。此外,肠道菌群的氨基酸代谢产物亚精胺可诱导肿瘤自噬、提高抗癌免疫反应并抑制肿瘤生长[44];肠道菌群相关的维生素B6则可协同顺铂等化疗药物杀伤非小细胞肺癌细胞,共同介导免疫依赖的抗肿瘤作用,改善晚期肺癌患者的新陈代谢和免疫[45]。肠道微生物群可影响肿瘤对免疫治疗的反应,提高肠道微生物β多样性进而改善肺癌患者的病理进程[46]。综上所述,调节肠道菌群和补充维生素可提高宿主抗癌免疫反应进而有效缓解肺癌,但目前关于肠道菌群与肺癌的早期诊断和治疗的研究较为有限,仍需更进一步探索。
2.2.3 肠道菌群与哮喘病 哮喘是一种以慢性气道炎症、气道高反应性和可逆性气道重塑为主要特征的异质性疾病[47]。研究发现,哮喘的发生与免疫调节密切相关,肠道菌群可通过调节辅助性T细胞1/辅助性T细胞2平衡,影响树突状细胞、Ⅱ型固有淋巴样细胞和辅助性T细胞17功能从而干预哮喘病的发生[48]。此外,哮喘患儿较健康人群相比体内毛螺菌属、韦荣氏菌属和罗氏菌属的菌群数量比例明显较低,链球菌属和拟杆菌属相对丰度显著上升[49]。上述研究表明,肠道菌群在哮喘病的诊断中发挥重要作用。
肠道菌群失衡与哮喘病的发生密切相关,可通过调节肠道菌群平衡为防治哮喘病探寻新的研究策略。VAEL 等[50]和ARRIETA等[51]研究表明,在哮喘发病过程中,肠道菌群结构和丰度的变化是导致哮喘发病的重要因素之一。与正常人相比,哮喘患者肠内毛螺菌属和罗氏菌属丰度下降,双歧杆菌和厌氧菌丰度增多。实验研究表明,肠道菌群代谢物短链脂肪酸能通过抑制由第2组先天淋巴细胞驱动的气道高反应性,诱导嗜酸性粒细胞介导的炎性反应,增加肠道淋巴结和脾脏中的调节性T细胞(regulatory T cells,Tregs)数量和活性来缓解哮喘病[7]。这一研究提示,短链脂肪酸可有效减少哮喘病患者的肺部炎症反应。此外,对哮喘患者补充可溶性膳食纤维可调整饮食结构减少其嗜酸性粒细胞和组蛋白去乙酰化酶的表达,降低促炎细胞因子水平,改善肺功能和气道反应亢进,从而延缓哮喘[52]。如上所述,短链脂肪酸可通过降低气道高反应性和上调Tregs细胞活性改善哮喘患者的机体炎症反应,从而延缓哮喘病的发生。
2.2.4 肠道菌群与慢性肺阻塞疾病 慢性肺阻塞疾病的发生与遗传、炎症反应和肺部发育异常密切相关[53]。慢性肺阻塞疾病患者肠道菌群不同于健康人群,包括链球菌、链霉菌在内的不同菌属都与患者肺功能减退有关[54]。此外,慢性肺阻塞疾病患者菌群多样性增加,放线菌门和变形菌门相对丰度增加,而拟杆菌门和厚壁菌门相对丰度降低且比例呈下降态势[55]。
“肠-肺轴”循环可引发肠黏膜屏障功能紊乱导致肠道菌群移位至血液甚至肺部,是急性呼吸窘迫综合征病发的主要原因[25]。而肺部的肺炎链球菌和流感嗜血菌可激活组织细胞丝裂原活化蛋白激酶MAPK,提高炎性细胞因子活化,并增加肠道通透性导致菌群异位通过血液流经肺部,进而导致急性呼吸窘迫综合征的发生[36]。有研究显示,肠道菌群丰度和多样性的变化是导致炎症反应的重要因素[56]。动物实验表明,戈登氏菌属和嗜黏蛋白阿克曼菌属等有益菌在慢性肺阻塞疾病小鼠模型中维持肠道菌群稳态能有效抑制链球菌属繁殖,改善辅助性T细胞17/Tregs细胞水平、降低肿瘤坏死因子α、白细胞介素1β和基质金属蛋白酶9的表达从而减轻肺部过敏性炎症[8]。与健康组对比,慢性肺阻塞疾病患者体内肠道菌群代谢物短链脂肪酸含量和拟杆菌门等有益菌呈明显下降趋势。果蔬和膳食纤维的补充可降低肺部拟杆菌门/厚壁菌门的比例,增加肠道和血液中短链脂肪酸含量,促进Tregs细胞分化并通过转录调控抑制树状突细胞功能来延缓慢性肺阻塞疾病发病[57]。此外,研究者们建立慢性肺阻塞疾病动物模型发现,粪肠球菌和屎肠球菌等致病菌快速生长会加剧慢性肺阻塞疾病患者机体炎症反应,而益生菌能通过提高机体固有免疫力加强自然杀伤细胞活性,使得肠和肺部免疫球蛋白A依赖型活化,抑制呼吸道嗜酸粒细胞产生,从而缓解气道敏感度[58-59]。另有研究表明,益生菌补充可抑制中性粒细胞等促炎因子的迁移和维持肠道菌群稳态来改善肺部炎症和损伤[60]。
上述研究提示,肠道菌群与慢性肺阻塞疾病的发生发展具有高度相关性,肠道菌群失调会加剧炎症反应和降低菌群多样性,从而诱发慢性肺阻塞疾病。膳食纤维和益生菌的补充可维持肠道菌群稳态和减缓气道炎症,有利于增强机体固有免疫能力,在防治呼吸道感染和减轻慢性肺阻塞疾病炎症中发挥有益效益。
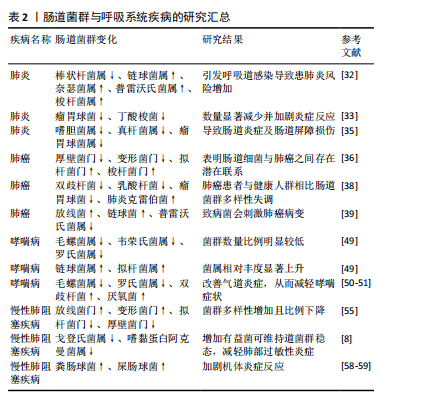
肠道菌群与呼吸系统疾病相关文献的汇总,见表2。
2.3 运动与肠道菌群
2.3.1 有氧运动对肠道菌群的影响 有氧运动也称为耐力性运动,是指是大肌群节律性、中等或较小强度、持续时间较长(10-60 min)的动力性运动,如骑自行车、太极、跑步和游泳等项目[61]。MOTLANI等[62]对糖尿病患者进行为期2周的自行车运动干预,发现短期有氧运动可降低厚壁菌/拟杆菌比值,减轻肿瘤坏死因子α表达和改善胰岛素敏感性。QUEIPO-ORTU?O等[63]对大鼠进行6 d的转轮运动,发现大鼠肠道厚壁菌门的乳杆菌属和真杆菌属以及放线菌门的双歧杆菌属含量与肠道菌群多样性呈显著上升趋势。TORQUATI等[64]对2型糖尿病患者进行8周有氧训练,发现双歧杆菌属、嗜黏蛋白阿克曼菌属、毛螺菌属和肠球菌属的丰度显著升高。徐山茸等[65]发现为期12周的走跑交替练习的高强度间歇性训练能增加拟杆菌丰度并降低厚壁菌丰度。另有研究发现,中等耐力游泳2周后,大鼠回肠中紧密连接蛋白1和闭锁小带蛋白1的mRNA水平升高,表明有氧运动可能会降低肠道通透性[66]。此外,郑贞等[67]研究发现,太极拳能提升不同年龄人群体内双歧杆菌丰度从而抑制炎症反应。
上述研究提示,有氧运动可改善胰岛素敏感性、调节肠道菌群丰度、改善肠屏障功能和缓解炎症反应对肠道菌群产生影响。但不同有氧运动形式对肠道菌群的影响也存在差异,这可能与运动强度以及受试者年龄、性别和健康程度有关。
2.3.2 抗阻运动对肠道菌群的影响 抗阻运动是指肌肉在“缺氧”的状态下短时间内快速收缩进行高速剧烈的运动,如短跑、举重和肌力训练等[61]。研究发现,肠道菌群代谢物短链脂肪酸中的丁酸盐可以通过G蛋白偶联受体43-蛋白激酶B-糖原合成酶激酶-3信号通路维持血糖稳定从而提高运动耐力表现[68]。抗阻运动可增加短链脂肪酸的生成改善肠道微生物群的多样性和组成[69]。YEH等[70]对雄性小鼠进行为期4周的抗阻运动并补充植物乳杆菌PL-02,发现小鼠肠道中的α多样性显著增加,并能缓解运动疲劳以及改善肌肉质量和运动表现。CHEN等[71]发现,抗阻运动能减少小鼠肠道固有层中白细胞介素17中T细胞的产生并增加Tregs细胞活化。最新研究表明,雄性大鼠进行抗阻训练并补充富含维生素D和亮氨酸的益生菌后,发现运动调节肠道菌群可有效改善超氧化物歧化酶的活性并提升总抗氧化能力[72]。另有研究发现,短乳酸杆菌可以增加氨基丁酸γ的产生从而增加血浆生长激素浓度,促进氨基酸转运、细胞氨基酸摄取和骨骼肌生长,同时抗阻运动联合短乳酸杆菌补充能增强健康人群机体的肌肉质量、提高力量表现和降低体脂率[73]。
2.3.3 有氧联合抗阻运动对肠道菌群的影响 陈海燕等[74]对8周龄雄性小鼠采用有氧联合抗阻运动干预,发现联合运动可增加肠道菌群种类和丰度,调节肠道菌群组成,提高肠道菌群多样性,其中小鼠肠内厚壁菌门的相对丰度均上升,变形菌门和拟杆菌门的相对丰度比例下降,属水平上毛螺菌属的相对丰度提高。韦薇等[75]发现为期8周的有氧联合抗阻运动能显著提高小鼠肠道中罗氏菌属和瘤胃球菌属的相对丰度。另有研究表明,2型糖尿病患者通过进行有氧联合抗阻运动,发现患者体内疣微菌门和放线菌门、双歧杆菌属和大肠杆菌属丰度升高[76]。目前运动对肠道菌群及其代谢物的研究多为单一有氧运动或者抗阻运动,尚缺乏针对有氧联合抗阻运动对肠道菌群数量和结构的报道,且运动调节肠道菌群的机制尚不明确,需进一步深入研究。
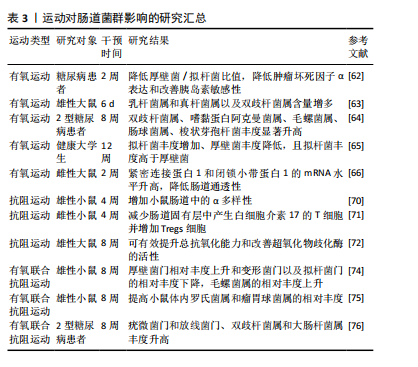
运动对肠道菌群影响相关文献的汇总,见表3。
2.4 运动与呼吸系统疾病 先前的研究已证明,运动可通过缓解炎症反应以及提升身体机能改善呼吸系统疾病。WANG等[77]研究发现,有氧运动可通过上调支气管肺泡灌洗液中的白细胞介素10和趋化因子1水平,并下调转化生长因子β和肿瘤坏死因子α水平,进而激活沉寂信息调节因子表达,缓解小鼠炎症反应和减轻氧化应激损伤,有效改善慢性肺阻塞疾病小鼠的肺气肿、支气管黏液细胞增生和肺纤维化等症状。MORAES-FERREIRA等[78]发现为期12周的有氧运动能调节哮喘患者炎症反应和维持纤维化介质的平衡,并改善患者慢性气道炎症。另有研究显示,肺炎患者进行每组8-10次的抗阻运动,发现患者步态速度、平衡和下肢力量等身体机能有所改善[79]。
HWANG等[80]研究发现,为期8周的有氧联合抗阻运动能显著改善晚期非小细胞肺癌化疗患者的肺功能,增加患者每分通气量和潮气量,进而缓解呼吸困难。此外,肺泡渗出增加和肺泡氧合减少是肺炎患者肺功能发生改变的重要病理机制,而中医药联合运动训练会有效改善新型冠状病毒肺炎愈后患者的肺功能、调节身心状态和提高患者的呼吸功能及运动能力,并降低其焦虑和抑郁状态[81]。上述研究提示,联合运动对呼吸系统疾病患者改善肺功能和提高生活质量具有重要意义。
2.5 运动调控肠道菌群在呼吸系统疾病中的作用机制 肠道菌群与呼吸系统疾病密切相关,且合理的运动可作为非药物干预手段调节肠道菌群。因此,以肠道菌群为靶点抑制炎症反应、调节氧化应激、改善肠屏障通透性和维持肠道菌群稳态等途径在防治呼吸系统疾病中发挥重要作用。
2.5.1 运动调控肠道菌群抑制炎症反应 炎症反应的发生是呼吸系统疾病的重要病理特征,因此抑制炎症反应是改善呼吸系统疾病的重要方式。ARRIETA等[82]通过建立气道炎症小鼠模型,将哮喘患儿的菌群定植到无菌小鼠体内,发现毛螺菌属、韦荣氏球菌属、普拉梭菌和罗斯氏菌属丰度增加,能有效改善无菌小鼠气道炎症。有氧运动可通过优化肠道菌群组成结构,增加肠道菌群代谢物短链脂肪酸(乙酸、丙酸、丁酸)数量[83],而短链脂肪酸经肠道吸收后可通过淋巴和血液系统转运到肺从而降低肺气道炎症和免疫反应,抑制组蛋白脱乙酰酶刺激促炎M1型巨噬细胞向抗炎M2型转化,下调核因子κB信号通路表达和促进白细胞介素10在中性粒细胞中的表达,影响促炎和抗炎机制平衡,进而改善过敏性气道炎症[84]。另有试验表明,女性耐力游泳运动员进行为期8周的有氧运动并摄入由嗜酸乳杆菌、保加利亚乳杆菌、双歧杆菌和嗜热链球菌组成的益生菌,发现运动可减轻炎症反应并增强对上呼吸道感染的抵抗力和改善免疫功能[85]。由此表明,有氧运动可调节肠道菌群并减轻炎症反应从而达到防治呼吸系统疾病的目的。
2.5.2 运动调控肠道菌群调节氧化应激 氧化应激在呼吸系统疾病的病理进程中具有重要作用。TANG等[86]研究表明,肠道菌群失衡可能调节肺免疫系统中的Toll样受体4/核因子κB信号通路,激活肺中的氧化应激引发炎症反应,并通过调节肠道屏障介导呼吸系统疾病。这一研究提示,肠道菌群调节氧化应激平衡可在防治呼吸系统疾病中发挥有益效应。此外,大强度及剧烈运动会导致活性氧迅速增加诱发氧化应激,破坏肠上皮细胞间的紧密连接状态,但补充益生菌可调节肠道菌群组成结构和降低氧化应激标志物水平[87-88]。另有研究发现,羽毛球、田径和自行车等项目的精英运动员服用瑞士乳杆菌Lafti L10补充剂可提高机体免疫力,抑制氧化应激损伤从而缩短运动员呼吸道感染持续时间[89-90]。上述研究共同表明,运动干预可靶向调节肠道菌群组成,促进肠道有益菌群丰度增加,从而抑制氧化应激水平延缓呼吸系统疾病的发生发展。
2.5.3 运动调控肠道菌群改善肠屏障通透性 肠道通透性的增加会导致肠道细菌及其代谢物转运到肺,进而引发呼吸系统疾病[91-93]。研究表明,中低强度运动通过调节紧密连接蛋白,改善肠黏膜屏障通透性影响肠道菌群组成,并通过上调分泌型免疫球蛋白A和下调促炎细胞因子来维持肠黏膜免疫稳态[94]。LUO等[66]对小鼠进行为期1周(每天30 min)中等强度游泳后发现,有氧运动干预能提高小肠抗菌肽基因表达,并减轻慢性应激诱导的肠屏障功能障碍。研究发现,阿克曼菌属减少与肠道通透性有关[95]。MUNUKKA等[96]对19名超重女生进行为期6周自行车运动干预后,发现受试者体内阿克曼菌属显著增加。此外,对从事耐力型体力活动的男性和女性进行为期4个月冬季训练并补充益生菌,发现干酪乳杆菌通过与肠道上皮细胞、M细胞和树突状细胞上的受体以及与普通黏膜免疫系统相互作用能改善肠黏膜通透性从而有效预防呼吸道感染[97]。
2.5.4 运动调控肠道菌群维持肠道菌群稳态 KHAILOVA等[98]建立肺炎小鼠模型,发现鼠李糖乳杆菌治疗可通过增强肠道黏蛋白屏障形成,减少细胞凋亡和改善细胞增殖来维持肠道屏障稳态,从而调节肺部的炎症反应和稳态。定期运动能增加微生物多样性,促进核苷酸代谢、葡萄糖代谢和脂质代谢相关菌群丰度,维持老年人肠道微生物群稳定性,从而为改善呼吸系统疾病提供新的治疗途径[75,99]。KNOLL等[100]对囊性纤维化肺病患者进行为期12个月的心肺运动测试并补充鼠李糖乳杆菌,发现患者体内肠道菌群多样性和丰富度保持稳定,因此定期锻炼可延缓其疾病进展并保持肺功能稳定。CLEMENTE等[101]发现,有氧运动或抗阻运动会影响拟杆菌门、厚壁菌门和变形菌门的相对丰度,提高肺功能并促进心肺健康,但这一研究受运动持续时间和强度的影响,仍需深入探索。上述研究提示,运动干预可通过维持肠道菌群稳态保持肺功能健康,进而改善呼吸系统疾病。

| [1] WHEELDON A. The respiratory system and associated disorders. Br J Nurs. 2023;32(2):613-619. [2] GUO X, OKPARA ES, HU W, et al. Interactive Relationships between Intestinal Flora and Bile Acids. Int J Mol Sci. 2022;23(7):8343. [3] LI C, LIU H, LIN Y, et al. The Gut Microbiota and Respiratory Diseases: New Evidence. J Immunol Res. 2020;17(2):1-12. [4] ALCAZAR CGM, PAES VM, SHAO Y, et al. The association between early-life gut microbiota and childhood respiratory diseases: a systematic review. Lancet Microbe. 2022;4(3):e867-e880. [5] RASTOGI S, MOHANTY S, SHARMA S, et al. Possible role of gut microbes and host’s immune response in gut–lung homeostasis. Front Immunol. 2022;13:954339. [6] WU Y, BISWAS D, USAITE I, et al. A local human Vδ1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat Cancer. 2022;3(2):696-709. [7] 唐徐韵,陈盼碧,杜狄佳,等.穴位埋线对哮喘大鼠肺组织中p38MAPK信号通路及细胞间黏附分子-1、白细胞介素-4和嗜酸性粒细胞的影响[J].针刺研究,2022,47(2):129-134 [8] WANG Y, LI N, LI Q, et al. Xuanbai Chengqi Decoction Ameliorates Pulmonary Inflammation via Reshaping Gut Microbiota and Rectifying Th17/Treg Imbalance in a Murine Model of Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2021;16(3):3317-3335. [9] SUN W, ZHOU T, DING P, et al. Bibliometric analysis of intestinal microbiota and lung diseases. Front Cell Infect Microbiol. 2024;14(2): 1347110. [10] KAHHALEH FG, BARRIENTOS G, CONRAD ML. The gut‐lung axis and asthma susceptibility in early life. Acta Physiol (Oxf). 2024;240(3): E14092. [11] SHI H, ZHAO T, GENG R, et al. The associations between gut microbiota and chronic respiratory diseases: a Mendelian randomization study. Front Microbiol. 2023;14(2):1200937. [12] LONGO S, RIZZA S, FEDERICI M. Microbiota-gut-brain axis: relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 2023;60(3):1007-1017. [13] COLELLA M, CHARITOS IA, BALLINI A, et al. Microbiota revolution: How gut microbes regulate our lives. World J Gastroentero. 2023; 29(2):4368-4383. [14] WANG Q, YANG Q, LIU X. The microbiota–gut–brain axis and neurodevelopmental disorders. Protein Cell. 2023;14(7):762-775. [15] WU R, XIONG R, LI Y, et al. Gut microbiome, metabolome, host immunity associated with inflammatory bowel disease and intervention of fecal microbiota transplantation. J Autoimmun. 2023;141:103062. [16] MAN MA, UNGUR RA, MOTOC NS, et al. Lung Microbiota in Idiopathic Pulmonary Fibrosis, Hypersensitivity Pneumonitis, and Unclassified Interstitial Lung Diseases: A Preliminary Pilot Study. Diagnostics. 2023; 13:3157. [17] WEI W, WANG S, XU C, et al. Gut microbiota, pathogenic proteins and neurodegenerative diseases. Front Microbiol. 2022;13:959856. [18] ZHANG Y, CHEN X, WANG Y, et al. Alterations of lower respiratory tract microbiome and short-chain fatty acids in different segments in lung cancer: a multiomics analysis. Front Cell Infect Microbiol. 2023; 13:1261284. [19] SINGH S, NATALINI JG, SEGAL L. Lung microbial-host interface through the lens of multi-omics. Mucosal Immunol. 2022;15: 837-845. [20] YIN Z, LIU X, GUO L, et al. The potential of dietary fiber in building immunity against gastrointestinal and respiratory disorders. Crit Rev Food Sci Nutr. 2023;9(3):1-19. [21] HUNGATE RE. Studies on Cellulose Fermentation. J Bacteriol. 1944;48: 499-513. [22] SCHAEDLER R, DUBOS R, COSTELLO R. Association of germfree mice with bacteria isolated from normal mice. J Exp Med. 1965;122:77-82. [23] QIN J, LI R, RAES J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [24] HILTY M, BURKE C, PEDRO H, et al. Disordered Microbial Communities in Asthmatic Airways. PLoS ONE. 2010;5:e8578. [25] VITAL M, HARKEMA J, RIZZO M, et al. Alterations of the Murine Gut Microbiome with Age and Allergic Airway Disease. J Immunol Res. 2015;2015:892568. [26] MJÖSBERG J, RAO A. Lung inflammation originating in the gut. Science. 2018;359(6371):36-37. [27] WYPYCH TP, WICKRAMASINGHE LC, MARSLAND B. The influence of the microbiome on respiratory health. Nat Immunol. 2019;20(10): 1279-1290. [28] NATALINI JG, SINGH S, SEGAL L. The dynamic lung microbiome in health and disease. Nat Rev Microbiol. 2022;21(4):222-235. [29] VINCENZO FD, GAUDIO AD, PETITO V, et al. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2023;19(2):275-293.
[30] CHEN H, LI S, PAN B, et al. Qing-Kai-Ling oral liquid alleviated pneumonia via regulation of intestinal flora and metabolites in rats. Front Microbiol. 2023;14:1194401. [31] ZHAO W, REN Z, LUO Y, et al. Metagenomics analysis of the gut microbiome in healthy and bacterial pneumonia forest musk deer. Genes Genomics. 2021;43(1):43-53. [32] THIBEAULT C, SUTTORP N, OPITZ B. The microbiota in pneumonia: From protection to predisposition. Sci Transl Med. 2021;13(576):EABA0501. [33] JIANG Y, BAO C, ZHAO X, et al. Intestinal bacteria flora changes in patients with Mycoplasma pneumoniae pneumonia with or without wheezing. Sci Rep. 2022;12(1):5683. [34] GIERSE L, MEENE A, SKORKA SB, et al. Impact of Pneumococcal and Viral Pneumonia on the Respiratory and Intestinal Tract Microbiomes of Mice. Microbiol Spectr. 2023;11(3):e0344722. [35] 钱文娟. “银翘”药对治疗H1N1肺炎的肺—肠轴相关代谢组学研究[D].南京:南京中医药大学,2019 [36] DICKSON R, SINGER B, NEWSTEAD M, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113. [37] BIRAGYN A, FERRUCCI L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018; 19(6):E295-E304. [38] POPE JL, TOMKOVICH S, YANG Y, et al. Microbiota as a mediator of cancer progression and therapy. Transl Res. 2017;179:139-154. [39] ZHANG WQ, ZHAO SK, LUO JW, et al. Alterations of fecal bacterial communities in patients with lung cancer. Am J Transl Res. 2018;10(10): 3171-3185. [40] SAINT-CRIQ V, LUGO-VILLARINO G, THOMAS M. Dysbiosis, malnutrition and enhanced gut-lung axis contribute to age-related respiratory diseases. Ageing Res Rev. 2020;66:101235. [41] ARPAIA N, CAMPBELL C, FAN X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature. 2013;504(7480):451-455. [42] WU Y, BISWAS D, USAITE I, et al. A local human Vδ1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat Cancer. 2022;3(6):696-709. [43] TANOUE T, MORITA S, PLICHTA D, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019; 565(7741):600-605. [44] PIETROCOLA F, POL JG, VACCHELLI E, et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell. 2016;30(1):147-160. [45] ARANDA F, BLOY N, PESQUET J, et al. Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene. 2014;34(23):3053-3062. [46] SONG P, YANG D, WANG H, et al. Relationship between intestinal flora structure and metabolite analysis and immunotherapy efficacy in Chinese NSCLC patients. Thorac Cancer. 2020;11(6):1621-1632. [47] HAMMAD H, LAMBRECHT BN. The basic immunology of asthma. Cell. 2021; 184(9):2521-2522. [48] 崔天怡,刘佳蕊,吕彬,等.肠道菌群及免疫调节与儿童哮喘关系的研究进展[J].中国全科医学,2022,25(8):1021-1026 [49] ARRIETA MC, STIEMSMA LT, DIMITRIU PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307RA152. [50] VAEL C, NELEN V, VERHULST SL, et al. Early intestinal Bacteroides fragilis colonisation and development of asthma. BMC Pulm Med. 2008;8:19. [51] ARRIETA MC, STIEMSMA LT, AMENYOGBE N, et al. The Intestinal Microbiome in Early Life: Health and Disease. Front Immunol. 2014; 5:427. [52] MCLOUGHLIN R, BERTHON B, ROGERS G, et al. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine. 2019; 46:473-485. [53] CHRISTENSON SA, SMITH BM, BAFADHEL M, et al. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227-2242. [54] BOWERMAN K, REHMAN SF, VAUGHAN A, et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat Commun. 2020;11(1):5886. [55] WANG L, CAI Y, GARSSEN J, et al. The Bidirectional Gut-Lung Axis in COPD. Am J Respir Crit Care Med. 2023;207(9):1145-1160. [56] WANG C, XU J, YANG L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018; 391(10131):1706-1717. [57] BLANCO-PÉREZ F, STEIGERWALD H, SCHÜLKE S, et al. The Dietary Fiber Pectin: Health Benefits and Potential for the Treatment of Allergies by Modulation of Gut Microbiota. Curr Allergy Asthma Rep. 2021;21(10):43. [58] JAMALKANDI SA, AHMADI A, AHRARI I, et al. Oral and nasal probiotic administration for the prevention and alleviation of allergic diseases, asthma and chronic obstructive pulmonary disease. Nutr Res Rev. 2020;34(1):1-16. [59] KAGEYAMA Y, NISHIZAKI Y, AIDA K, et al. Lactobacillus plantarum induces innate cytokine responses that potentially provide a protective benefit against COVID‑19: A single‑arm, double‑blind, prospective trial combined with an in vitro cytokine response assay. Exp Ther Med. 2021;23(1):20. [60] CARVALHO J, MIRANDA M, FIALHO AK, et al. Oral feeding with probiotic Lactobacillus rhamnosus attenuates cigarette smoke-induced COPD in C57Bl/6 mice: Relevance to inflammatory markers in human bronchial epithelial cells. PLOS ONE. 2019;15(4):e0225560. [61] 运动处方中国专家共识(2023)[J].中国运动医学杂志,2023,42(1): 3-13 [62] MOTIANI KK, COLLADO MC, COLLADO MC, et al. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med Sci Sports Exerc. 2019;52(1):94-104. [63] QUEIPO-ORTUÑO MI, SEOANE L, MURRI M, et al. Gut Microbiota Composition in Male Rat Models under Different Nutritional Status and Physical Activity and Its Association with Serum Leptin and Ghrelin Levels. PLoS ONE. 2013;8(5):e65465. [64] TORQUATI L, GAJANAND T, COX E, et al. Effects of exercise intensity on gut microbiome composition and function in people with type 2 diabetes. Eur J Sport Sci. 2022;23(4):530-541. [65] 徐山茸,龚莉,储文文,等.12周高强度间歇性训练对人体肠道菌群的影响[J].微生物学通报,2021,48(4):1215-1226 [66] LUO B, XIANG D, NIEMAN D, et al. The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense. Brain Behav Immun. 2014;39:99-106. [67] 郑贞,罗杨,鄢显明,等.不同训练时间太极拳运动康复方案对肠道菌群功能的影响[J].中国病原生物学杂志,2020,15(9):1071-1074 [68] ZHANG WQ, ZHAO TT, GUI DK, et al. Sodium Butyrate Improves Liver Glycogen Metabolism in Type 2 Diabetes Mellitus. J Agric Food Chem. 2019;67(27):7694-7705.
[69] BYCURA DK, SANTOS AC, SHIFFEr A, et al. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports. 2021;9(2):14.
[70] YEH WL, HSU YJ, HO CS, et al. Lactobacillus plantarum PL-02 Supplementation Combined With Resistance Training Improved Muscle Mass, Force, and Exercise Performance in Mice. Front Nutr. 2022;9: 896503. [71] CHEN H, SHEN L, LIU Y, et al. Strength Exercise Confers Protection in Central Nervous System Autoimmunity by Altering the Gut Microbiota. Front Immunol. 2021:12:628629. [72] MOHABBAT M, ARAZI H. Effect of resistance training plus enriched probiotic supplement on sestrin2, oxidative stress, and mitophagy markers in elderly male Wistar rats. Sci Rep. 2024;14(1):7744. [73] LEE MC, HSU YJ, HO CS, et al. Supplementation with Lactiplantibacillus brevis GKEX Combined with Resistance Exercise Training Improves Muscle Mass, Strength Performance, and Body Fat Condition in Healthy Humans. Foods. 2024;13(7):1030. [74] 陈海燕,张迷磊,莫彬彬,等.基于肠道菌群探讨运动对2型糖尿病小鼠血糖的影响[J].广西医科大学学报,2023,40(10):1699-1707. [75] 韦薇,张秋,黄燕凤,等.不同运动方式对2型糖尿病小鼠肠道菌群及短链脂肪酸的影响[J].广西医科大学学报,2022,39(4):643-648. [76] LAMPRECHT M, FRAUWALLNER A. Exercise, Intestinal Barrier Dysfunction and Probiotic Supplementation. Med Sport Sci. 2012;59: 47-56. [77] WANG X, WANG Z, TANG D. Aerobic Exercise Alleviates Inflammation, Oxidative Stress, and Apoptosis in Mice with Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2021;16:1369-1379. [78] MORAES-FERREIRA R, BRANDAO-RANGEL MAR, GIBSON-ALVES TG, et al. Physical Training Reduces Chronic Airway Inflammation and Mediators of Remodeling in Asthma. Oxid Med Cell Longev. 2022; 2022:5037553. [79] UDINA C, ARS J, MORANDI A, et al. Rehabilitation in adult post-COVID-19 patients in post-acute care with Therapeutic Exercise. J Frailty Aging. 2021;10(3):297-300 [80] HWANG CL, YU CJ, SHIH JY, et al. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer. 2012;20(12):3169-3177. [81] 王娜,余兴艳,秦利春,等.老年新冠肺炎愈后患者中医药治疗联合运动康复训练方法及应用效果[J].中华医院感染学杂志,2024, 34(7):1016-1020. [82] ARRIETA MC, STIEMSMA LT, DIMITRIU PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. [83] SOHAIL M, YASSINE H, SOHAIL A, et al. Impact of Physical Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic Disorders. Rev Diabet Stud. 2019;15:35-48. [84] JARDOU M, LAWSON R. Supportive therapy during COVID-19: The proposed mechanism of short-chain fatty acids to prevent cytokine storm and multi-organ failure. Med Hypotheses 2021;154:110661 [85] SALARKIA N, GHADAMLI L, ZAERI F, et al. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: a randomized controlled trial. Med J Islam Repub Iran. 2013;27(3):141-146. [86] TANG JF, XU L, ZENG Y, et al. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int Immunopharmacol. 2020;91:107272. [87] DÍAZ-JIMÉNEZ J, SÁNCHEZ-SÁNCHEZ E, ORDOÑEZ FJ, et al. Impact of Probiotics on the Performance of Endurance Athletes: A Systematic Review. Int J Environ Res Public Health. 2021;18(21):11576. [88] ZEPPA SD, AGOSTINI D, GERVASI M, et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients. 2019;12(1):17. [89] LAMPRECHT M, BOGNER S, SCHIPPINGER G, et al. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012;9(1):45. [90] MICHALIČKOVÁ D, MINIĆ R, DIKIĆ N, et al. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2016;41(7):782-789. [91] BINGULA R, FILAIRE M, RADOSEVIC-ROBIN N, et al. Desired Turbulence? Gut-Lung Axis, Immunity, and Lung Cancer. J Oncol. 2017: 2017:5035371. [92] MCALEER J, KOLLS J. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. 2018;48(1):39-49 [93] TROMPETTE A, GOLLWITZER E, PATTARONI C, et al. Dietary Fiber Confers Protection against Flu by Shaping Ly6c−Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity. 2018;48(5): 992-1005 [94] 吴嵽,陈佩杰,罗贝贝.运动对肠道屏障和黏膜免疫稳态影响的研究进展[J].体育科学,2018,38(6):67-75 [95] EVERARD A, BELZER C, GEURTS L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066-9071 [96] MUNUKKA E, AHTIAINEN JP, PUIGBÒ P, et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front Microbiol. 2018;9:2323. [97] GLEESON M, BISHOP N, OLIVEIRA M, et al. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab. 2011;21(1):55-64 [98] KHAILOVA L, BAIRD CH, RUSH AA, et al. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates inflammatory response and homeostasis of spleen and colon in experimental model of Pseudomonas aeruginosa pneumonia. Clin Nutr. 2017;36(6):1549-1557 [99] MENON M, HUSSELL T, SHUWA HA. Regulatory B cells in respiratory health and diseases. Immunol Rev. 2021;299(1):61-73 [100] KNOLL RL, JARQUÍN-DÍAZ VH, KLOPP J, et al. Resilience and stability of the CF- intestinal and respiratory microbiome during nutritional and exercise intervention. BMC Microbiology. 2023;23(1):44 [101] CLEMENTE F, BRAVINI E, CORNA S, et al. The relationship between physical exercise and gut microbiota in the human being: a systematic review. Epidemiol Prev. 2021;45(4):245-253. |
| [1] | 于经邦, 吴亚云. 非编码RNA在肺纤维化过程中的调控作用[J]. 中国组织工程研究, 2025, 29(8): 1659-1666. |
| [2] | 王秋月, 靳 攀, 蒲 锐. 运动干预与细胞焦亡在骨关节炎中的作用[J]. 中国组织工程研究, 2025, 29(8): 1667-1675. |
| [3] | 袁维勃, 刘 婵, 余丽梅. 肝脏类器官在肝脏疾病模型与移植治疗中的应用潜力[J]. 中国组织工程研究, 2025, 29(8): 1684-1692. |
| [4] | 赵晓璇, 刘帅祎, 李 奇, 邢 政, 李庆雯, 褚晓蕾. 不同运动方式促进周围神经损伤后的功能恢复[J]. 中国组织工程研究, 2025, 29(6): 1248-1256. |
| [5] | 纪 龙, 陈子扬, 靳 攀, 孔祥魁, 蒲 锐, . 脂肪自噬、运动干预与非酒精性脂肪肝的防治[J]. 中国组织工程研究, 2025, 29(35): 7611-7619. |
| [6] | 汪 涛, 王顺谱, 闵友江, 王 敏, 李 乐, 张 宸, 肖伟平. 肠道菌群与类风湿关节炎的因果关系:GWAS数据欧洲群体资料分析[J]. 中国组织工程研究, 2025, 29(35): 7663-7668. |
| [7] | 王佳倩, 蒋昌君, 彭 毅, 马 咪, 李军汉. 有氧运动对CNPY2基因调控AKT/GSK3β通路改善非酒精性脂肪肝的作用研究[J]. 中国组织工程研究, 2025, 29(30): 6441-6448. |
| [8] | 刘昊为, 田浩冬, 黄 丽, 余杭林, 彭 莉. 血流限制抗阻运动对肥胖青年男性血清代谢物的急性影响[J]. 中国组织工程研究, 2025, 29(29): 6249-6259. |
| [9] | 何宁娟, 李 丽, 王 素, 杨建设, 雷思韵, 王 扬. 有氧或抗阻运动对阿尔茨海默病小鼠海马Ras/Drebrin树突棘可塑性的影响[J]. 中国组织工程研究, 2025, 29(26): 5528-5535. |
| [10] | 沙拉依丁·艾尔西丁, 艾克拜尔江·艾赛提, 库提鲁克·守克尔, 古丽米热·依力哈木, 艾克热木江·木合热木. 外周神经轴突损伤中沃勒变性的细胞生物学机制#br#[J]. 中国组织工程研究, 2025, 29(26): 5688-5694. |
| [11] | 耿珑玉, 盛 黎, 白 硕, 高蓓瑶, 葛瑞东, 江 山. 细胞焦亡在运动系统疾病中的作用及相关分子机制[J]. 中国组织工程研究, 2025, 29(26): 5695-5703. |
| [12] | 蒋千平, 杨 丹, 万石磊, 徐丹丹, 曹 璐, 周 晶, . O连接N-乙酰葡萄糖胺糖基化在神经退行性疾病中的作用及临床应用前景[J]. 中国组织工程研究, 2025, 29(26): 5704-5712. |
| [13] | 赵 鹏, 王聪聪, 王晨宇. 有氧运动对心肌梗死患者内皮祖细胞动员和功能的影响[J]. 中国组织工程研究, 2025, 29(23): 4947-4955. |
| [14] | 杨 朔, 张 振, 白 硕, 盛 黎, 申 亮, 孙青峰, 高蓓瑶, 葛瑞东, 江 山. 线粒体功能障碍与肌腱病:靶向线粒体治疗的可能性[J]. 中国组织工程研究, 2025, 29(20): 4276-4285. |
| [15] | 胡淑娟, 程 平, 张 啸, 丁一庭, 刘 璇, 蒲 锐, 汪献旺. 不同强度运动干预2型糖尿病大鼠骨骼肌羧酸酯酶1及炎症因子的变化[J]. 中国组织工程研究, 2025, 29(2): 269-278. |
展[2]。有研究发现,肠道菌群失调与呼吸系统疾病密切相关[3-4]。调节肠道菌群多样性、维持肠屏障功能完整性和抑制炎症反应可增强机体免疫能力,进而延缓呼吸系统疾病的病理进程[5-8]。近年来,随着“肠-肺轴”和“肠-脑轴”概念的提出,肠道菌群与肺炎、肺癌、哮喘病和慢性肺阻塞性疾病等呼吸系统疾病之间的关联引起广泛关注[9-11]。
研究发现,肠道菌群丰度和多样性的变化可刺激机体达到免疫状态从而影响呼吸系统疾病的发生发展。此外,运动也可通过降低促炎因子表达、抑制氧化应激水平、改善肠屏障功能障碍和维持肠道菌群稳态调节肠道菌群。但现阶段关于运动调节肠道菌群防治呼吸系统疾病的作用机制尚未明晰,不同运动方式和运动强度对其具体影响仍需进一步研究。因此,文章综述了肠道菌群与肺炎、肺癌、哮喘病和慢性肺阻塞疾病等不同呼吸系统疾病之间的作用机制以及运动对肠道菌群和呼吸系统疾病的影响,并深入探讨运动调控肠道菌群在呼吸系统疾病中的分子机制,梳理总结了肠道菌群与呼吸系统疾病及运动干预三者间的相互作用关系,以期为运动防治呼吸系统疾病提供新的思路和理论依据。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 第一作者于2023年9月至2024年5月进行文献检索收集。
1.1.2 检索词 中文检索词为“肠道菌群、肠道细菌、呼吸系统疾病、肺炎、肺癌、哮喘病、慢性肺阻塞疾病、有氧运动、抗阻运动”,英文检索词为“Intestinal flora,Gut bacteria,Respiratory illness,Pneumonia,Lung cancer,
Asthma,Chronic Obstructive pulmonary diseases,Aerobic exercise、Resistance training”。
1.1.3 检索文献时限 检索1944-2024年发表的相关文献。
1.1.4 检索数据库 CNKI和PubMed数据库。
1.1.5 检索文献类型 期刊文章(研究原著、综述等)和学位论文。
1.1.6 检索策略 以PubMed 数据库为例,见表1。
1.1.7 检索文献量 经过综合检索,共检索到文献2 566篇,其中中文文献480篇、英文文献2 086篇。
1.2 资料筛选流程和筛选标准
1.2.1 纳入标准 ①肠道菌群与呼吸系统疾病的相关研究;②运动与肠道菌群的相关研究;③运动与呼吸系统疾病的相关研究;④肠道菌群与呼吸系统疾病及运动干预的相关研究。
1.2.2 排除标准 ①排除与该文无关文献;②排除会议和重复性研究。
1.3 检索流程 见图1。
3.1 既往他人在该领域研究的贡献及存在问题 多年来,呼吸系统疾病的发病机制错综复杂,关于其具体机制尚未被详细阐述。但随着“肠-肺轴”概念的提出,研究者们发现肠道菌群与呼吸系统疾病之间密切相关。肠道菌群可通过药物干预、饮食和菌群定植等多种方式调控肠道菌群改善呼吸系统疾病,且有研究表明运动可有效改善肠道菌群组成和多样性、抑制炎症反应、调节氧化应激、改善肠屏障通透性和维持肠道菌群稳态,从而在防治呼吸系统疾病中发挥有益效益。但当前研究仍存在以下问题:①现阶段由于研究受技术限制,关于肠道菌群在呼吸系统疾病中的具体作用机制仍有待进一步深入研究;②近年来有关综述主要围绕运动介导肠道菌群维持机体健康展开论述,对于呼吸系统疾病的研究鲜有报道;③临床试验较少,制约了肠道菌群在呼吸系统疾病治疗中的广泛应用。
3.2 作者综述区别于他人他篇的特点 既往研究多关注肠道菌群在呼吸系统疾病中的变化,该综述以肠道菌群为切入点,较为全面系统地梳理总结了近年来运动调控肠道菌群防治呼吸系统疾病的研究进展,探讨不同运动形式如有氧运动、抗阻运动和有氧联合抗阻运动对肠道菌群的影响进行总结分析,并突出合理运动可维持肠道菌群平衡进而发挥防治呼吸系统疾病的潜在作用,为促进机体健康以及呼吸系统疾病的防治提供新思路。
3.3 综述的局限性 迄今为止,多数研究集中在肠道菌群作为各种病理生物标志物的作用上。但目前关于肠道菌群对呼吸系统疾病的相互作用尚未阐明,肠道菌群与呼吸系统疾病间深层机制研究仍需进一步探索。此外,大部分肠道菌群研究仍停留在防治各类疾病的变化趋势层面,鲜有采用不同形式的运动进行深入探究,以进一步明确运动在肠道菌群中的具体调控作用。
3.4 综述的重要意义 文章一步总结了肠道菌群在肺炎、肺癌、哮喘病和慢性肺阻塞疾病等不同呼吸系统疾病的作用机制,为阐明肠道菌群与呼吸系统疾病的关系以及运动调控肠道菌群改善呼吸系统疾病的相关研究开辟新途径。运动可通过调控肠道菌群抑制炎症反应、调节氧化应激、改善肠屏障通透性和维持肠道菌群稳态等不同途径抑制炎性因子水平、减少氧化应激标志物、维持肠黏膜免疫平衡、调节机体稳态等过程促进肺健康,为呼吸系统疾病的干预与治疗提供新的靶点。随着高通量测序技术的不断发展,运动在调节肠道菌群防治呼吸系统疾病中发挥着至关重要的作用,为促进机体健康提供理论参考依据。
3.5 课题组专家的建议 ①肠道菌群与不同呼吸系统疾病间的联系有待更深层次的探讨;②不同运动方式、运动强度和运动时间影响肠道菌群的具体生物学机制及差异性仍需深入研究;③现阶段的实验干预以动物或细胞实验为主,尚缺乏更具体的临床试验证明其科学性,后续应从多维度、多元化和多视角的组合形式积极探索为呼吸系统疾病的治疗开辟新方向;④虽然大量研究已证实肠道菌群与呼吸系统疾病及运动干预三者间存在紧密关联,但具体关系仍有待进一步揭示。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||