中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (19): 4122-4131.doi: 10.12307/2025.069
• 干细胞综述 stem cell review • 上一篇 下一篇
不同干细胞来源外泌体及其携非编码RNA诊疗骨性关节炎
王 喆,齐岩松,徐永胜
- 内蒙古自治区人民医院骨科中心(运动医学中心),内蒙古自治区呼和浩特市 010017
-
收稿日期:2024-02-21接受日期:2024-04-28出版日期:2025-07-08发布日期:2024-09-13 -
通讯作者:徐永胜,博士,主任医师,内蒙古自治区人民医院骨科中心(运动医学中心),内蒙古自治区呼和浩特市 010017 -
作者简介:王喆,女,1996年生,山东省青岛市人,汉族,内蒙古医科大学在读硕士,主要从事运动医学临床与基础方向的研究。 通讯作者:齐岩松,博士,副主任医师,副研究员,内蒙古自治区人民医院骨科中心(运动医学中心),内蒙古自治区呼和浩特市 010017 -
基金资助:国家自然科学基金面上项目(82172444),项目负责人 :徐永胜
Diagnosis and treatment of osteoarthritis with exosomes derived from different stem cells and carrying non-coding RNA
Wang Zhe, Qi Yansong, Xu Yongsheng
- Orthopedic Center (Sports Medicine Center) of Inner Mongolia Autonomous Region People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China
-
Received:2024-02-21Accepted:2024-04-28Online:2025-07-08Published:2024-09-13 -
Contact:Xu Yongsheng, MD, Chief physician, Orthopedic Center (Sports Medicine Center) of Inner Mongolia Autonomous Region People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China -
About author:Wang Zhe, Master candidate, Orthopedic Center (Sports Medicine Center) of Inner Mongolia Autonomous Region People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China. Corresponding author: Qi Yansong, MD, Associate chief physician, Associate researcher, Orthopedic Center (Sports Medicine Center) of Inner Mongolia Autonomous Region People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China -
Supported by:National Natural Science Foundation of China (General Program), No. 82172444 (to XYS)
摘要:
文题释义:
外泌体:是由细胞分泌携带各种细胞成分的细胞外囊泡,包括DNA、RNA、脂质、代谢物以及胞质和细胞表面蛋白,与免疫反应、病毒致病性、妊娠、心血管疾病、中枢神经系统相关疾病和癌症等多种生理和病理过程相关。非编码RNA:指由基因组转录而成的不编码蛋白质的 RNA 分子。
摘要
背景:外泌体可在骨性关节炎患者滑液和血浆中检测出来,其水平随着骨性关节炎的进展而不断变化,并且可以对骨性关节炎的局部炎症、软骨钙化和关节退化起缓解作用。
目的:旨在全面了解不同干细胞来源外泌体在骨性关节炎诊疗中的作用和机制,并提出了外泌体治疗骨性关节炎的前景和挑战。
方法:检索PubMed及中国知网数据库,英文检索词为“exosomes,osteoarthritis,mesenchymal stem cells,stem cells”,中文检索词为“外泌体,骨性关节炎,间充质干细胞,干细胞”,对2003年10月至2023年10月发表的文献进行检索,最终纳入99篇文献进行综述。
结果与结论:外泌体的出现给骨性关节炎的诊断和治疗带来了希望。外泌体内含RNA、蛋白质及脂类的差异能够作为骨性关节炎的生物标志物用于诊断。同时,来自不同干细胞的外泌体均能够有效保护软骨细胞、缓解炎症、维持软骨基质代谢以及调节血管生成和软骨下骨重塑,表现出治疗骨性关节炎的优秀潜力。工程化外泌体则突破传统局限,通过调节特定非编码RNA表达增强治疗特异性与效率,为骨性关节炎的治疗提供新策略。
https://orcid.org/0009-0007-9339-6620(王喆);https://orcid.org/0000-0003-1764-493X(齐岩松);
https://orcid.org/0009-0006-9931-9864(徐永胜)
中图分类号:
引用本文
王 喆, 齐岩松, 徐永胜. 不同干细胞来源外泌体及其携非编码RNA诊疗骨性关节炎[J]. 中国组织工程研究, 2025, 29(19): 4122-4131.
Wang Zhe, Qi Yansong, Xu Yongsheng. Diagnosis and treatment of osteoarthritis with exosomes derived from different stem cells and carrying non-coding RNA[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4122-4131.
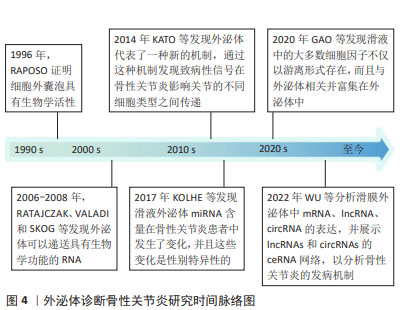
迄今为止,从血液或滑液中分离的外泌体已被确定能够有效诊断骨性关节炎[24],外泌体中的核酸,如:信使RNA(messenger RNA,mRNA)、微小RNA(microRNA,miRNA)、lncRNA、环状RNA (circular RNA,circRNA)均在骨性关节炎患者外泌体中存在差异表达。WU等[25]发现骨性关节炎组与对照组滑膜外泌体中有52个mRNA、196个lncRNA和98个circRNA表达存在差异,它们可能在骨性关节炎病理机制中发挥重要作用。骨性关节炎软骨细胞外泌体miRNA表达水平存在差异[16],骨性关节炎患者滑液中的外泌体miRNA与非骨性关节炎组存在差异并具有性别差异表达,男性与女性分别有114与144个miRNA存在表达差异[26]。
特定外泌体miRNA的表达在关节疾病患者中发生改变[27]。研究发现,破骨细胞可以通过外泌体将miRNA转移到软骨细胞上,从而改变软骨细胞的状态,降低其对软骨基质变性和血管神经侵犯的抵抗力,最终导致骨性关节炎的进一步发展[28]。KOLHE等[26]发现女性骨性关节炎患者滑液的细胞外囊泡中靶向免疫和Toll样受体相关基因的miRNA表达减少,导致预防炎症的能力降低,这可能是女性骨性关节炎发病率较高的原因之一。MENG等[29]证实,与健康受试者相比,骨性关节炎患者血浆中外泌体miR-193b-3p的表达降低。正常滑膜外泌体促进软骨形成,骨性关节炎患者成纤维细胞样滑膜细胞分泌的外泌体中miR-19b-3p,miR-19b-3p通过海绵化骨性关节炎中的溶质载体家族成员SLC7A11来加剧软骨细胞铁死亡及软骨损伤[30]。CHEN等[31]证实,相比于健康人,骨性关节炎患者滑液中外泌体miR-130b-3p和miR-1271-5p显著上调,这两种miRNA可能在骨性关节炎细胞通讯中起关键作用,具有作为标志物对骨性关节炎进行诊断的潜力。
外泌体中的一些lncRNA也具有用作骨性关节炎生物标志物的可能性。ZHAO等[32]对血浆和滑液中的外泌体lncRNA进行了分析,发现血浆来源外泌体lncRNA的表达没有显著差异,但滑液来源外泌体lncRNA前列腺特异性转录本1的表达在骨性关节炎患者中显著升高,并且在骨性关节炎的不同阶段表达存在差异,在骨性关节炎晚期表达远高于骨性关节炎早期,可用于诊断骨性关节炎的进展程度。
CircRNA是一类具有环状结构和优异稳定性非编码RNA,随着近年来基因测序技术的快速发展,circRNA的各种作用被越来越多的探索。circRNA参与骨性关节炎的一系列重要病理生理过程,尤其是其竞争性内源RNA机制,在骨性关节炎中发挥着重要作用[33]。FU等[34]发现骨性关节炎患者软骨组织中Circ_0128846表达明显上调,其通过miR-940/PTPN12通路调控骨性关节炎患者软骨细胞增殖、凋亡和炎症。血浆中circRNA-016901的表达与骨性关节炎患者疾病严重程度密切相关。骨性关节炎患者血浆中circRNA-016901的表达水平较高并在治疗后降低,而在类风湿性关节炎、骨坏死及健康对照组中未观察到血浆circRNA-016901的改变,可利用circRNA-016901在血浆中表达程度对骨性关节炎进行诊断[35]。由于circRNA内部结构高度稳定,且存在于外泌体及细胞外囊泡中,具有表达模式高度特异性和半衰期较长的特点,可作为诊断骨性关节炎的良好工具。
此外,外泌体中的蛋白质或脂类也可用作生物标志物。滑液中的细胞因子不仅游离存在,还富集于外泌体中,晚期骨性关节炎患者滑液外泌体中的细胞因子如白细胞介素1β、白细胞介素17、白细胞介素10和干扰素水平明显高于早期骨性关节炎患者[36]。KOLHE等[37]发现骨性关节炎患者外泌体中多种蛋白存在性别特异性差异,女性骨性关节炎患者外泌体中的结合珠蛋白、α-酸性糖蛋白及铜蓝蛋白的表达显著上调,男性骨性关节炎患者外泌体中β-2-糖蛋白和补体C5的表达显著上调。
外泌体在骨性关节炎早期阶段可被检测到,对于早期诊断具有重要意义。通过研究骨性关节炎患者和健康人滑液和血浆等体液来源外泌体中RNA、蛋白质及脂类等物质的差异,能够预测易感人群,外泌体有希望作为骨性关节炎早期快速诊断工具。目前外泌体对于疾病诊治处于“瓶颈期”,在未来有希望利用外泌体中所含RNA、蛋白质及脂类在骨性关节炎进展过程中发生变化的特点,实时了解病情的发展,评估骨性关节炎的治疗效果并调整制定治疗方案。
2.2 不同干细胞来源外泌体治疗骨性关节炎
2.2.1 骨髓间充质干细胞来源外泌体(bone mesenchymal stem cell-derived exosomes,BMSC-Exos) 骨髓间充质干细胞是来源于骨髓的基质干细胞,是一种具有分化为成骨、脂肪、软骨等多种分化潜能的细胞亚群。现有研究已证明,BMSC-Exos能够促进成骨和血管生成[38],同时能够极大地促进受损软骨和软骨下骨的修复[39],对骨性关节炎具有良好的治疗效果。
BMSC-Exos可以有效促进骨性关节炎软骨修复和合成。BMSC-Exos通过上调白细胞介素1β诱导的Ⅱ型胶原α1和聚集蛋白聚糖的表达以及下调基质金属蛋白酶13和含血小板反应蛋白解整合素金属肽酶5(a disintegrin and metalloproteinase with thrombospondin motifs 5,ADAMTS5)的表达,显著消除了白细胞介素1β对软骨细胞增殖和迁移的抑制作用[40]。JIN等[41]通过横断前交叉韧带和内侧半月板失稳术建立大鼠骨性关节炎模型后,在关节内注射骨髓间充质干细胞或其外泌体。BMSC-Exos能够减轻白细胞介素1β诱导的ADAMTS5、基质金属蛋白酶13和Ⅱ型胶原α1表达的改变以及通过lncRNA MEG-3抑制白细胞介素1β诱导的软骨细胞衰老和凋亡;体内实验发现,BMSC-Exos可减轻骨性关节炎大鼠关节损伤,恢复骨小梁体积分数、骨小梁数量和连接密度。骨髓间充质干细胞和BMSC-Exos均可缓解骨性关节炎大鼠软骨破坏和软骨下骨重塑,BMSC-Exos可显著抑制骨性关节炎大鼠背根神经节组织中降钙素基因相关肽和诱导型一氧化氮合酶表达的上调,从而缓解骨性关节炎的疼痛[40]。
外泌体的治疗作用主要是通过过表达各种非编码RNA完成的,包括miRNAs、 lncRNAs、circRNAs等,近年来研究人员逐渐重视对于外泌体中非编码RNA的研究。BMSC-Exos能够逆转白细胞介素1β诱导的细胞凋亡,其原因是其内含的lncRNA LYRM4-AS1通过调节GRPR-miR-6515-5p通路调控白细胞介素1β对软骨细胞的损伤从而缓解骨性关节炎炎症[42]。XU等[43]发现,BMSC-Exos可通过靶向HDAC3和STAT1/NF-κB p65,将外泌体中miR-326传递至软骨细胞和软骨,抑制软骨细胞的焦亡从而改善骨性关节炎。
BMSC-Exos能够通过多个通路影响软骨细胞的增殖、代谢及凋亡,进而促进软骨及软骨下骨的修复,起到延缓并治疗骨性关节炎的作用,并对骨性关节炎的疼痛具有良好的缓解效果。自然条件下获得的外泌体数量有限,且成分复杂多变,这限制了其在临床应用中的广泛使用。工程化外泌体直接利用细胞分泌的天然外泌体装载蛋白或者核酸等药物,通过工程化技术可以优化外泌体的载荷,使其能够高效携带和递送更多种类的治疗分子,增强对于骨性关节炎的治疗效果。
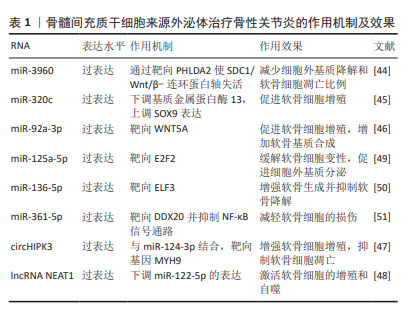
BMSC-Exos可以通过负载miR-3960来上调Ⅱ型胶原和聚集蛋白聚糖的表达,同时下调ADAMTS5和基质金属蛋白酶13的表达,以减少细胞外基质降解和软骨细胞凋亡比例[44]。SUN等[45]发现,过表达miR-320c的BMSC-Exos在促进骨关节炎软骨细胞增殖、下调基质金属蛋白酶13和上调SOX9表达方面相比未过表达特定基因的外泌体更为有效。过表达miR-92a-3p的BMSC-Exos通过靶向WNT5A,抑制WNT信号通路,促进软骨增殖,增加软骨基质合成[46]。LI等[47]证明过表达circHIPK3的BMSC-Exos可以通过与miR-124-3p结合,然后靶向基因MYH9来促进软骨细胞增殖,同时抑制软骨细胞凋亡。过表达lncRNA NEAT1能够下调miR-122-5p的表达,激活软骨细胞的增殖和自噬[48]。还有许多miRNA通过在外泌体中过表达能够有效缓解骨性关节炎,如: miR-125a-5p[49]、miR-136-5p[50]、miR-361-5p等[51]。见表1。
特定细胞基质的预处理也能够提高外泌体的治疗效果。脱细胞基质(decellularized extracellular matrix,dECM)为间充质干细胞的扩增提供了最佳的微环境。ZHANG等[52]发现,相比于BMSC-Exos,脱细胞基质预处理的BMSC-Exos(dECM-BMSC-Exos)靶向磷酸酯酶与张力蛋白同源物,通过上调miR-3473b的表达来促进软骨细胞的增殖、合成代谢、迁移和抗凋亡等特性,并具有更好的软骨再生效果,从而促进骨性关节炎的缓解。
BMSC-Exos能够通过多个通路影响软骨细胞的增殖、代谢及凋亡,进而促进软骨及软骨下骨的修复,起到延缓并治疗骨性关节炎的作用,并对骨性关节炎的疼痛具有良好的缓解效果。通过开发工程化外泌体携带特定RNA能够显著增强BMSC-Exos对于骨性关节炎的治疗效果。虽然BMSCs-Exos对于改善骨性关节炎具有良好的效果,但由于骨髓间充质干细胞的提取需要进行骨髓穿刺等侵入性手术,在临床应用的转化中受到一定限制。
2.2.2 滑膜间充质干细胞来源外泌体(synovial mesenchymal stem cell-derived exosomes,SMSC-Exos) 滑膜间充质干细胞能够通过传递外泌体促进软骨细胞增殖、迁移、Ⅱ型胶原α1和聚集蛋白聚糖的表达,同时抑制细胞凋亡[53]。GUO等[54]发现,SMSC-Exos可以被骨髓间充质干细胞摄取并增强其增殖及抗凋亡能力。此外,SMSC-Exos可以显著抑制地塞米松对骨髓间充质干细胞增殖的抑制作用,这表明SMSC-Exos可以用于联合治疗以提高治疗效果。
研究表明,SMSC-Exos中富含Wnt5a和Wnt5b,Wnt5a和Wnt5b通过激活Wnt信号通路,促进Yes相关蛋白的激活,从而增强软骨细胞的增殖和迁移能力。同时,Wnt5a和Wnt5b激活后抑制SOX9的表达,从而抑制细胞外基质的合成[55-56]。TAO等[57]发现,过表达miR-140-5p的SMSCs-Exos(SMSC-140-Exos)将增强关节软骨细胞的增殖和迁移能力而不会损害细胞外基质的分泌。研究人员对SMSC-Exos应用过表达miR-155-5p进行修饰,研究发现,SMSC-155-5p-Exos在体外促进骨性关节炎软骨细胞的增殖和迁移、抑制细胞凋亡,并通过结合位点负向调节Runt相关转录因子2(Runt-related transcription factor 2,Runx2)的表达增强细胞外基质分泌[58]。Runx2在骨性关节炎滑膜组织中上调,并与miR-155-5p呈负相关。体外实验证明,SMSC-155-5p-Exos在小鼠模型中可有效治疗骨性关节炎。过表达circRNA3503的SMSCs-Exos作为has-miR-181c-3p和has-let-7b-3p的分子海绵,保持软骨细胞外基质合成与降解的平衡[59]。除过表达特定RNA外,应用含脂多糖的培养基对SMSCs-Exos进行预处理也能够通过抑制细胞外基质降解,提高外泌体疗效[60]。
负载特定RNA能够增强SMSC-Exos减轻软骨细胞炎症的能力,ZHENG等[61]实验发现过表达miR-212-5p的SMSC-Exos(SMSC-212-5p-Exos)通过抑制白细胞介素1β诱导的软骨细胞中E74样ETS转录因3(E74 like ETS transcription factor 3,ELF3)的表达上调,缓解白细胞介素1β诱导的软骨细胞变性、降解和炎症反应。此外, SMSC-Exos过表达miR-129-5p也可以通过抑制HMGB1的释放来减轻白细胞介素1β诱导的骨性关节炎[62]。
外泌体也可通过过表达相应RNA改善软骨损伤。KONG等[53]研究表明,过表达miR-302c的SMSCs-Exos通过靶向去整合素和金属蛋白酶(A Disintegrin and Metallo-proteinases 19, ADAM19)促进软骨形成。过表达miR-320c的SMSCs-Exos通过降低ADAM19以及Wnt信号传导的2个关键蛋白β-catenin和MYC的水平(即靶向ADAM19依赖的Wnt信号通路),抑制骨性关节炎大鼠细胞外基质降解和软骨细胞凋亡,促进软骨损伤修复。相比于滑膜间充质干细胞及未过表达miR-320c的SMSCs-Exos可更好降低内侧半月板失稳术大鼠骨性关节炎国际研究学会(OARSI)评分,促进软骨损伤修复,抑制软骨炎症、细胞外基质降解和软骨细胞凋亡[63]。LU等[64]在体内验证了含有miR-26a-5p的SMSCs-Exos通过直接靶向PTEN基因抑制软骨凋亡及减轻软骨损伤。
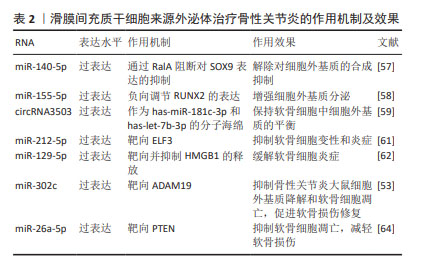
针对SMSC-Exos增强骨性关节炎软骨细胞的增殖和迁移、抑制细胞凋亡,但会导致细胞外基质分泌不足的问题,研究人员利用过表达特定miRNA、circRNA或脂多糖预处理来解除合成抑制及增强合成分泌。特定RNA的过表达也能够增强SMSC-Exos减轻软骨细胞炎症、缓解软骨损伤的能力,对骨性关节炎具有更好的治疗效果。见表2。
2.2.3 脂肪间充质干细胞来源外泌体(adipose mesenchymal stem cell-derived exosomes,ADSC-Exos) 脂肪间充质干细胞是来源于脂肪组织的干细胞,脂肪组织是人体最丰富的组织之一,这使得脂肪间充质干细胞具有来源丰富和获取相对容易的优势,是一种较为理想的细胞来源。脂肪间充质干细胞可以分化为软骨细胞,并分泌多种生长因子,如骨形态发生蛋白和成纤维细胞生长因子等,这些生长因子能够促进软骨再生和修复,这使得脂肪间充质干细胞成为一种治疗骨性关节炎的有效手段,关节内注射自体脂肪间充质干细胞能够显著改善骨性关节炎患者关节功能、缓解疼痛[65]。
ADSC-Exos通过旁分泌作用促进软骨再生、缓解炎症反应[66]。ZHAO等[67]研究发现ADSC-Exos通过上调miR-145和miR-221的表达,能够促进软骨形成并抑制炎症。此外,ADSC-Exos还能下调促炎标志物白细胞介素6、NF-κB和肿瘤坏死因子α的表达、上调抗炎细胞因子白细胞介素10的表达以及阻止H2O2诱导的软骨细胞凋亡。
ADSC-Exos经过修饰能达到更好地治疗骨性关节炎的效果。过表达miR-376c-3p的ADSC-Exos通过靶向WNT3或WNT9a抑制Wnt/β-catenin通路,减轻骨性关节炎导致的软骨细胞凋亡和滑膜纤维化[68]。miR-338-3p修饰的ADSC-Exos通过抑制Runx2表达可有效修复白细胞介素1β诱导的软骨细胞改变,对于骨性关节炎具有良好的缓解作用[69]。过表达miR-429的ADSC-Exos促进软骨细胞增殖,并通过靶向 FEZ2 促进自噬来改善骨关节炎的软骨损伤[70]。负载miR-199a-3p的ADSC-Exos能够促进骨性关节炎动物模型的软骨形成并抑制软骨细胞凋亡,保护软骨的同时改善步态障碍[71]。
内质网应激参与骨性关节炎中的软骨细胞凋亡和软骨退变[72]。外源性miR-486-5p可抑制内质网应激,同时能够减轻软骨细胞凋亡以及促进基质再生。WANG等[73]验证了过表达miR-486-5p的ADSC-Exos与直接使用miR-486-5p相比,前者能够更好地缓解软骨细胞凋亡以治疗骨性关节炎。
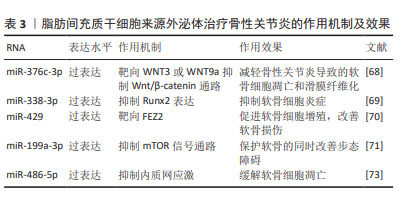
ADSC-Exos对软骨细胞的迁移和增殖具有强烈的刺激作用,同时能够很好地缓解软骨细胞凋亡及骨性关节炎软骨损伤,在人体实验中表现出良好的治疗效果。由于脂肪间充质干细胞可以通过患者特异性的方式获得,并且理论上是取之不尽的, ADSC-Exos可能是未来非常有希望应用于临床的治疗手段。见表3。
2.2.4 人脐带间充质干细胞来源外泌体(human umbilical cord mesenchymal stem cell-derived exosomes,hucMSC- Exos) 人脐带间充质干细胞由脐带组织中提取,hucMSC- Exos在促进软骨细胞增殖和迁移以及抑制软骨细胞凋亡方面有良好功效[74-75],可以逆转白细胞介素1β诱导的软骨细胞损伤,并在体外调节巨噬细胞的极化[76]。研究表明,人脐带间充质干细胞能够安全有效治疗骨性关节炎[77],并且与BMSC-Exos相比,hucMSC-Exos具有更为良好的治疗效果[78]。
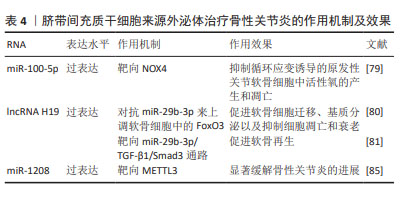
LI等[79]研究发现,hucMSC-Exos通过miR-100-5p靶向NOX4,抑制循环应变诱导的原发性关节软骨细胞中活性氧的产生和凋亡。hucMSC-Exos可以作为lncRNA H19的天然载体,并且外泌体中高水平的lncRNA H19可以促进软骨细胞增殖并防止细胞凋亡[80]。YAN等[81]发现过表达lncRNA H19的hucMSC-Exos作为竞争性内源RNA对抗miR-29b-3p来上调软骨细胞中的FoxO3,促进软骨细胞迁移、基质分泌以及抑制细胞凋亡和衰老。王宪峰等[82]发现,过表达lncRNA H19的hucMSC-Exos通过靶向miR-29b-3p/TGF-β1/Smad3通路促进软骨再生。
NOD样受体热蛋白结构域相关蛋白3(NOD-like receptor thermal protein domain associated protein 3,NLRP3)是巨噬细胞炎症的重要组成部分,已被确定为骨性关节炎的潜在新型生物标志物,与诱导具有促进软骨退变和滑膜炎症功能的白细胞介素1β和白细胞介素18分泌相关[83-84]。
ZHOU等[85]证明来源于人脐带间充质干细胞的细胞外囊泡(hucMSC-EVs)通过miR-1208靶向甲基转移样酶3(methyltransferase like 3,METTL3)来抑制NLRP3 mRNA甲基化,从而抑制炎症因子释放、Ⅱ型胶原α1和聚集蛋白聚糖降解以及ADAMTS3和基质金属蛋白酶13的过表达,显著缓解小鼠膝骨性关节炎的进展。
hucMSC-Exos能够促进软骨再生,显著缓解骨性关节炎进展。hucMSCs具有易于提取且具有巨大的分化潜能的特性,脐带是临床上的医疗废物,不会对捐赠者造成伤害,因此其在治疗骨性关节炎方面具有很大的潜力。见表4。
2.2.5 牙髓干细胞来源外泌体(dental pulp stem cell-derived exosomes,DPSC-Exos) DPSC-Exos能够促进软骨细胞相关mRNA的表达,包括聚集蛋白聚糖、Ⅱ型胶原α1和SOX9。FU等[86]在小鼠膝骨性关节炎模型中单次关节内注射DPSC-Exos,发现有效改善了异常软骨下骨重塑,抑制了骨硬化和骨赘的发生,缓解了体内软骨退化和滑膜炎症,其机制为DPSC-Exos通过抑制瞬时受体电位香草酸通道4(transient receptor potential vanilloid 4,TRPV4)活化来抑制体内破骨细胞活化,这可能是临床骨性关节炎治疗的有希望的靶点。LIN等[87]发现富含miR-140的DPSC-Exos可能通过调节细胞凋亡相关蛋白的表达水平发挥抗凋亡作用,从而改善骨性关节炎大鼠模型的膝关节症状。
目前针对DPSC-Exos负载特定RNA进行成牙或成骨分化的研究较为多见,基于DPSC-Exos能够有效改善软骨下骨重塑的特点,工程化DPSC-Exos是一个具有前景与希望的课题。
2.2.6 羊水干细胞来源外泌体(amniotic fluid stem cell-derived exosomes,AFSC-Exos) 羊水干细胞已被证明可以分泌包含生长因子和免疫调节分子的外泌体,阻止组织降解并诱导软骨修复。BERETTI等[88]发现,AFSC-Exos与羊水干细胞存在不同的分子,其中一些分子参与免疫调节,如转化生长因子和肝细胞生长因子。此外,AFSC-Exos中存在正五聚蛋白,这是一类调节炎症反应的蛋白质,可以诱导巨噬细胞极化,抑制炎症并预防纤维化。ZAVATTI等[89]证明AFSCs-Exos可以对抗软骨损伤,并发现其效果与转化生长因子β含量呈正相关。
2.2.7 胚胎间充质干细胞来源外泌体(embryonic mesenchymal stem cell-derived exosomes,EMSC-Exos) 胚胎间充质干细胞是从人胚胎干细胞分化出来的间充质干细胞,可以治疗多种自身免疫和炎症性疾病,其疗效与自体细胞组织(如骨髓)来源间充质干细胞相似。与体细胞来源的间充质干细胞相比,胚胎间充质干细胞可以很容易地通过基因组编辑进行修饰,并且可以大规模生产[90]。
ZHANG等[91]研究表明,每周关节内给予人EMSC-Exos 可促进成年免疫功能正常大鼠骨软骨缺损的修复。WANG等[92]也发现关节内注射胚胎间充质干细胞减轻了内侧半月板失稳术小鼠模型的软骨破坏和基质降解。体外研究表明,EMSC-Exos通过增加Ⅱ型胶原合成和降低ADAMTS5表达,来平衡软骨细胞外基质的合成和降解以及阻止软骨破坏从而缓解骨性关节炎。
EMSCs的多能分化潜能和优异的软骨再生能力,给骨性关节炎的治疗带来了新的希望,然而涉及人类胚胎的伦理问题仍是阻碍EMSC-Exos进行临床应用的绊脚石。
2.2.8 诱导多能干细胞来源间充质干细胞外泌体(induced pluripotent stem cell-derived mesenchymal stem cell-derived exosomes,iPS-MSCs-Exos) 诱导多能干细胞是使用特定的转录因子从成人体细胞中重新编程的多能细胞,具有胚胎干细胞潜在的无限增殖的特征,并能够进行成骨和成骨诱导[93]。iPS-MSCs-Exos可以通过激活内皮细胞中PI3K/Akt信号通路,显著增强内皮细胞的增殖、迁移和成管能力[94]。诱导多能干细胞可以由患者特异性成体细胞诱导,由其衍生的间充质干细胞具有患者的特异性,理论上能够解决受体需要免疫抑制的问题。ZHU等[95]对比了SMSC- Exos和iPS-MSCs-Exos治疗骨性关节炎的疗效,相比于SMSC-Exos,iPS-MSCs-Exos具有更好的治疗效果,能够更加显著刺激软骨细胞的迁移和增殖。iPS-MSCs-Exos避免了伦理争议和免疫学问题,可能是未来再生医学更有前途的细胞来源。
2.2.9 其他干细胞来源的外泌体 人尿源性干细胞(human urine-derived stem cells,hUSCs)来源外泌体(hUSC-Exos)能够增强软骨细胞增殖和迁移能力,有效减少细胞凋亡,但会造成细胞外基质分泌减少。LIU等[96]发现,与hUSC-Exos相比,过表达miR-140-5p的hUSC-Exos(hUSC-140-Exos),不仅能使关节软骨细胞增殖和迁移能力进一步增强,而且通过靶向血管内皮生长因子A增加了细胞外基质的分泌;关节内注射hUSC-140-Exos可促进软骨再生、软骨下骨重塑和调节细胞外基质稳态,缓解骨性关节炎进展。
鹿角干细胞起源于神经嵴干细胞,具有强大的增殖和分化潜力[97-98]。LEI等[99]利用小鼠骨性关节炎模型发现鹿角干细胞来源外泌体可以减轻人类干细胞衰老与软骨变性。虽然鹿角干细胞来源外泌体研究时间尚短,但具有作为生物材料治疗骨性关节炎和其他年龄相关疾病的巨大潜力。
| [1] MARTEL-PELLETIER J, BARR AJ, CICUTTINI FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [2] HUNTER DJ, SCHOFIELD D, CALLANDER E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014; 10(7):437-441. [3] YUE L, BERMAN J. What Is Osteoarthritis? JAMA. 2022;327(13):1300. [4] BRANDT KD, RADIN EL, DIEPPE PA, et al. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65(10): 1261-1264. [5] YAO Q, WU X, TAO C, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):56. [6] CRAWFORD DC, MILLER LE, BLOCK JE. Conservative management of symptomatic knee osteoarthritis: a flawed strategy? Orthop Rev (Pavia). 2013;5(1):e2. [7] KESTER BS, MINHAS SV, VIGDORCHIK JM, et al. Total Knee Arthroplasty for Posttraumatic Osteoarthritis: Is it Time for a New Classification? J Arthroplasty. 2016;31(8):1649-1653.e1. [8] KALLURI R, LEBLEU VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [9] MATHIEU M, MARTIN-JAULAR L, LAVIEU G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9-17. [10] GYÖRGY B, SZABÓ TG, TURIÁK L, et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One. 2012;7(11):e49726. [11] HEADLAND SE, JONES HR, NORLING LV, et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7(315):315ra190. [12] DOMENIS R, ZANUTEL R, CAPONNETTO F, et al. Characterization of the Proinflammatory Profile of Synovial Fluid-Derived Exosomes of Patients with Osteoarthritis. Mediators Inflamm. 2017;2017: 4814987. [13] VERGUNST CE, VAN DE SANDE MG, LEBRE MC, et al. The role of chemokines in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 2005;34(6):415-425. [14] XIE F, LIU YL, CHEN XY, et al. Role of MicroRNA, LncRNA, and Exosomes in the Progression of Osteoarthritis: A Review of Recent Literature. Orthop Surg. 2020;12(3):708-716. [15] LIU Q, WANG R, HOU S, et al. Chondrocyte-derived exosomes promote cartilage calcification in temporomandibular joint osteoarthritis. Arthritis Res Ther. 2022;24(1):44. [16] KATO T, MIYAKI S, ISHITOBI H, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. [17] LU K, WANG Q, HAO L, et al. miR-204 ameliorates osteoarthritis pain by inhibiting SP1-LRP1 signaling and blocking neuro-cartilage interaction. Bioact Mater. 2023;26:425-436. [18] KAUSHIK S, CUERVO AM. Proteostasis and aging. Nat Med. 2015;21(12): 1406-1415. [19] FANG Y, NI J, WANG YS, et al. Exosomes as biomarkers and therapeutic delivery for autoimmune diseases: Opportunities and challenges. Autoimmun Rev. 2023;22(3):103260. [20] AHMAD A. Exosomes in Cancer Diagnosis and Therapy. Int J Mol Sci. 2022;23(17):9930. [21] LIU Z, LI Y, WANG Y, et al. Exosomes in HBV infection. Clin Chim Acta. 2023;538:65-69. [22] JOO HS, JEON HY, HONG EB, et al. Exosomes for the diagnosis and treatment of dementia. Curr Opin Psychiatry. 2023;36(2):119-125. [23] ALJUHANI A, ALBALAWI O, ALBALAWI R, et al. Exosomes in COVID-19 infection: Focus on role in diagnosis, pathogenesis, immunity, and clinical trials. Cell Biol Int. 2023;47(6):1049-1067. [24] YOUNG DA, BARTER MJ, SOUL J. Osteoarthritis year in review: genetics, genomics, epigenetics. Osteoarthritis Cartilage. 2022; 30(2):216-225. [25] WU X, BIAN B, LIN Z, et al. Identification of exosomal mRNA, lncRNA and circRNA signatures in an osteoarthritis synovial fluid-exosomal study. Exp Cell Res. 2022;410(1):112881. [26] KOLHE R, HUNTER M, LIU S, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):2029. [27] 孙硕,张锡光,岳乔宁,等.外泌体携载微小RNA治疗骨关节炎的研究[J].中国骨质疏松杂志,2023,29(2):248-251. [28] LIU J, WU X, LU J, et al. Exosomal transfer of osteoclast-derived miRNAs to chondrocytes contributes to osteoarthritis progression. Nat Aging. 2021;1(4):368-384. [29] MENG F, LI Z, ZHANG Z, et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics. 2018;8(10):2862-2883. [30] KONG R, JI L, PANG Y, et al. Exosomes from osteoarthritic fibroblast-like synoviocytes promote cartilage ferroptosis and damage via delivering microRNA-19b-3p to target SLC7A11 in osteoarthritis. Front Immunol. 2023;14:1181156. [31] CHEN P, RUAN A, ZHOU J, et al. Identification and analysis of key microRNAs derived from osteoarthritis synovial fluid exosomes. Chin Med J (Engl). 2023;136(2):245-247. [32] ZHAO Y, XU J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018; 42(12):2865-2872. [33] XUE Q, HUANG Y, CHANG J, et al. CircRNA-mediated ceRNA mechanism in Osteoarthritis: Special emphasis on circRNAs in exosomes and the crosstalk of circRNAs and RNA methylation. Biochem Pharmacol. 2023; 212:115580. [34] FU G, YIN F, ZHAO J. Depletion of circ_0128846 ameliorates interleukin-1β-induced human chondrocyte apoptosis and inflammation through the miR-940/PTPN12 pathway. Int Immunopharmacol. 2022;110:108996. [35] DU M, FAN S, LIU Y, et al. The Application of circRNA-016901 in Improving the Diagnostic Accuracy of Osteoarthritis. Biomed Res Int. 2022;2022:1158562. [36] GAO K, ZHU W, LI H, et al. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod Rheumatol. 2020;30(4):758-764. [37] KOLHE R, OWENS V, SHARMA A, et al. Sex-Specific Differences in Extracellular Vesicle Protein Cargo in Synovial Fluid of Patients with Osteoarthritis. Life (Basel). 2020;10(12):337. [38] ZHANG L, JIAO G, REN S, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11(1):38. [39] ASGHAR S, LITHERLAND GJ, LOCKHART JC, et al. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford). 2020;59(1):57-68. [40] HE L, HE T, XING J, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11(1):276. [41] JIN Y, XU M, ZHU H, et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J Cell Mol Med. 2021;25(19):9281-9294. [42] WANG X, LI Z, CUI Y, et al. Exosomes Isolated From Bone Marrow Mesenchymal Stem Cells Exert a Protective Effect on Osteoarthritis via lncRNA LYRM4-AS1-GRPR-miR-6515-5p. Front Cell Dev Biol. 2021;9:644380. [43] XU H, XU B. BMSC-Derived Exosomes Ameliorate Osteoarthritis by Inhibiting Pyroptosis of Cartilage via Delivering miR-326 Targeting HDAC3 and STAT1//NF-κB p65 to Chondrocytes. Mediators Inflamm. 2021;2021:9972805. [44] YE P, MI Z, WEI D, et al. miR-3960 from Mesenchymal Stem Cell-Derived Extracellular Vesicles Inactivates SDC1/Wnt/β-Catenin Axis to Relieve Chondrocyte Injury in Osteoarthritis by Targeting PHLDA2. Stem Cells Int. 2022;2022:9455152. [45] SUN H, HU S, ZHANG Z, et al. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem. 2019;120(1):171-181. [46] MAO G, ZHANG Z, HU S, et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9(1):247. [47] LI S, LIU J, LIU S, et al. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J Nanobiotechnology. 2021;19(1):343. [48] ZHANG S, JIN Z. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing Long Noncoding RNA NEAT1 Relieve Osteoarthritis. Oxid Med Cell Longev. 2022;2022:5517648. [49] XIA Q, WANG Q, LIN F, et al. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021;12(2):11225-11238. [50] CHEN X, SHI Y, XUE P, et al. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res Ther. 2020;22(1):256. [51] TAO Y, ZHOU J, WANG Z, et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg Chem. 2021;113:104978. [52] ZHANG Y, QI G, YAN Y, et al. Exosomes derived from bone marrow mesenchymal stem cells pretreated with decellularized extracellular matrix enhance the alleviation of osteoarthritis through miR-3473b/phosphatase and tensin homolog axis. J Gene Med. 2023;25(8):e3510. [53] KONG R, GAO J, ZHANG J, et al. Synovial mesenchymal stem cell-derived exosomal miR-320c enhances chondrogenesis by targeting ADAM19. Future Med Chem. 2022;14(2):81-96. [54] GUO SC, TAO SC, YIN WJ, et al. Exosomes from Human Synovial-Derived Mesenchymal Stem Cells Prevent Glucocorticoid-Induced Osteonecrosis of the Femoral Head in the Rat. Int J Biol Sci. 2016;12(10):1262-1272. [55] PARK HW, KIM YC, YU B, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162(4):780-794. [56] LIU CF, LEFEBVRE V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res. 2015;43(17):8183-8203. [57] TAO SC, YUAN T, ZHANG YL, et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180-195. [58] WANG Z, YAN K, GE G, et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol Toxicol. 2021;37(1):85-96. [59] TAO SC, HUANG JY, GAO Y, et al. Small extracellular vesicles in combination with sleep-related circRNA3503: A targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact Mater. 2021;6(12):4455-4469. [60] DUAN A, SHEN K, LI B, et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res Ther. 2021;12(1):427. [61] ZHENG T, LI Y, ZHANG X, et al. Exosomes Derived From miR-212-5p Overexpressed Human Synovial Mesenchymal Stem Cells Suppress Chondrocyte Degeneration and Inflammation by Targeting ELF3. Front Bioeng Biotechnol. 2022;10:816209. [62] QIU M, LIU D, FU Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. [63] KONG R, ZHANG J, JI L, et al. Synovial mesenchymal stem cell-derived exosomal microRNA-320c facilitates cartilage damage repair by targeting ADAM19-dependent Wnt signalling in osteoarthritis rats. Inflammopharmacology. 2023;31(2):915-926. [64] LU L, WANG J, FAN A, et al. Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A-26a-5p ameliorate cartilage damage of osteoarthritis. J Gene Med. 2021;23(11):e3379. [65] LEE WS, KIM HJ, KIM KI, et al. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med. 2019;8(6):504-511. [66] TOFIÑO-VIAN M, GUILLÉN MI, PÉREZ DEL CAZ MD, et al. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxid Med Cell Longev. 2017;2017:7197598. [67] ZHAO C, CHEN JY, PENG WM, et al. Exosomes from adipose‑derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR‑145 and miR‑221. Mol Med Rep. 2020;21(4): 1881-1889. [68] LI F, XU Z, XIE Z, et al. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA -376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis. 2023; 28(3-4):362-378. [69] LI C, LI W, PU G, et al. Exosomes derived from miR-338-3p-modified adipose stem cells inhibited inflammation injury of chondrocytes via targeting RUNX2 in osteoarthritis. J Orthop Surg Res. 2022;17(1):567. [70] MENG C, NA Y, HAN C, et al. Exosomal miR-429 derived from adipose-derived stem cells ameliorated chondral injury in osteoarthritis via autophagy by targeting FEZ2. Int Immunopharmacol. 2023;120:110315. [71] ZHAO S, XIU G, WANG J, et al. Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis. J Nanobiotechnology. 2023;21(1):341. [72] LIU Y, ZHU H, YAN X, et al. Endoplasmic reticulum stress participates in the progress of senescence and apoptosis of osteoarthritis chondrocytes. Biochem Biophys Res Commun. 2017;491(2):368-373. [73] WANG Y, FAN A, LU L, et al. Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem Pharmacol. 2022;206:115343. [74] MIANEHSAZ E, MIRZAEI HR, MAHJOUBIN-TEHRAN M, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10(1):340. [75] 郭天赐,刘爱峰,陈继鑫,等.人脐带源间充质干细胞源性外泌体在骨科疾病中的研究进展[J].河北医药,2023,45(1):125-130. [76] LI P, LV S, JIANG W, et al. Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann Transl Med. 2022;10(18):976. [77] 孙月,沈阳,刘铭,等.人脐带血间充质干细胞治疗膝关节小范围软骨缺损疗效研究[J].创伤与急危重病医学,2022,10(3):191-194. [78] 王宪峰,欧昕,邓必勇.不同来源间充质干细胞外泌体治疗骨关节炎疗效的比较[J].中国组织工程研究,2022,26(25):3980-3985. [79] LI X, WANG Y, CAI Z, et al. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol Int. 2021;45(10):2096-2106. [80] YAN L, LIU G, WU X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J Orthop Translat. 2020;26:111-120. [81] YAN L, LIU G, WU X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin Transl Med. 2021;11(1):e255. [82] 王宪峰,王锟,孙晗,等.脐带间充质干细胞外泌体LncRNA H19修复软骨损伤的机制[J].中国组织工程研究,2024,28(1):20-25. [83] SWANSON KV, DENG M, TING JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477-489. [84] JIN C, FRAYSSINET P, PELKER R, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci U S A. 2011;108(36):14867-14872. [85] ZHOU H, SHEN X, YAN C, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res Ther. 2022;13(1):322. [86] FU Y, CUI S, ZHOU Y, et al. Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. Int J Mol Sci. 2023;24(5):4926. [87] LIN T, WU N, WANG L, et al. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR‑140‑5p‑overexpressing human dental pulp stem cells. Int J Mol Med. 2021;47(3):7. [88] BERETTI F, ZAVATTI M, CASCIARO F, et al. Amniotic fluid stem cell exosomes: Therapeutic perspective. Biofactors. 2018;44(2):158-167. [89] ZAVATTI M, BERETTI F, CASCIARO F, et al. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. Biofactors. 2020;46(1):106-117. [90] JIANG B, FU X, YAN L, et al. Transplantation of human ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous osteoarthritis in rhesus macaques. Theranostics. 2019;9(22): 6587-6600. [91] ZHANG S, CHU WC, LAI RC, et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135-2140. [92] WANG Y, YU D, LIU Z, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017; 8(1):189. [93] KO JY, IM GI. Chondrogenic and Osteogenic Induction from iPS Cells. Methods Mol Biol. 2016;1357:441-450. [94] LIU X, LI Q, NIU X, et al. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int J Biol Sci. 2017;13(2):232-244. [95] ZHU Y, WANG Y, ZHAO B, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1):64. [96] LIU Y, ZENG Y, SI HB, et al. Exosomes Derived From Human Urine-Derived Stem Cells Overexpressing miR-140-5p Alleviate Knee Osteoarthritis Through Downregulation of VEGFA in a Rat Model. Am J Sports Med. 2022;50(4):1088-1105. [97] WANG Y, ZHANG C, WANG N, et al. Genetic basis of ruminant headgear and rapid antler regeneration. Science. 2019;364(6446):eaav6335. [98] WANG D, BERG D, BA H, et al. Deer antler stem cells are a novel type of cells that sustain full regeneration of a mammalian organ-deer antler. Cell Death Dis. 2019;10(6):443. [99] LEI J, JIANG X, LI W, et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2022;13(3):220-226. |
| [1] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 余 帅, 刘家伟, 朱 彬, 潘 檀, 李兴龙, 孙广峰, 于海洋, 丁 亚, 王宏亮. 小分子药物治疗骨关节炎的热点问题及应用前景[J]. 中国组织工程研究, 2025, 29(9): 1913-1922. |
| [3] | 赵济宇, 王少伟. 叉头框转录因子O1信号通路与骨代谢[J]. 中国组织工程研究, 2025, 29(9): 1923-1930. |
| [4] | 尹 路, 蒋川锋, 陈俊杰, 易 明, 王子赫, 石厚银, 汪国友, 沈骅睿. 沙苑子苷A对关节软骨细胞凋亡的影响[J]. 中国组织工程研究, 2025, 29(8): 1541-1547. |
| [5] | 于经邦, 吴亚云. 非编码RNA在肺纤维化过程中的调控作用[J]. 中国组织工程研究, 2025, 29(8): 1659-1666. |
| [6] | 袁维勃, 刘 婵, 余丽梅. 肝脏类器官在肝脏疾病模型与移植治疗中的应用潜力[J]. 中国组织工程研究, 2025, 29(8): 1684-1692. |
| [7] | 胡涛涛, 刘 兵, 陈 诚, 殷宗银, 阚道洪, 倪 杰, 叶凌霄, 郑祥兵, 严 敏, 邹 勇. 过表达神经调节蛋白1的人羊膜间充质干细胞促进小鼠皮肤创面愈合[J]. 中国组织工程研究, 2025, 29(7): 1343-1349. |
| [8] | 金 凯, 唐 婷, 李美乐, 谢裕安. 人脐带间充质干细胞条件培养基及外泌体对肝癌细胞增殖、迁移、侵袭和凋亡的影响[J]. 中国组织工程研究, 2025, 29(7): 1350-1355. |
| [9] | 李帝均, 酒精卫, 刘海峰, 闫 磊, 李松岩, 王 斌. 明胶三维微球装载人脐带间充质干细胞修复慢性肌腱病[J]. 中国组织工程研究, 2025, 29(7): 1356-1362. |
| [10] | 娄 国, 张 敏, 付常喜. 8周运动预适应增强脂肪干细胞治疗心肌梗死大鼠的效果[J]. 中国组织工程研究, 2025, 29(7): 1363-1370. |
| [11] | 刘 琪, 李林臻, 李玉生, 焦泓焯, 杨 程, 张君涛. 淫羊藿苷含药血清促进3种细胞共培养体系中软骨细胞增殖和干细胞成软骨分化[J]. 中国组织工程研究, 2025, 29(7): 1371-1379. |
| [12] | 艾克帕尔·艾尔肯, 陈晓涛, 吾凡别克·巴合提. 成骨诱导人牙周膜干细胞来源外泌体促进炎症微环境下人牙周膜干细胞成骨分化[J]. 中国组织工程研究, 2025, 29(7): 1388-1394. |
| [13] | 章镇宇, 梁秋健, 杨 军, 韦相宇, 蒋 捷, 黄林科, 谭 桢. 新橙皮苷治疗骨质疏松症的靶点及对骨髓间充质干细胞成骨分化的作用[J]. 中国组织工程研究, 2025, 29(7): 1437-1447. |
| [14] | 吕丽婷, 于 霞, 张金梅, 高巧婧, 刘仁凡, 李 梦, 王 璐. 脑衰老与外泌体研究进程及现状的文献计量学分析[J]. 中国组织工程研究, 2025, 29(7): 1457-1465. |
| [15] | 谢刘刚, 崔书克, 郭楠楠, 李遨宇, 张菁瑞. 干细胞治疗阿尔茨海默病的研究热点与前沿[J]. 中国组织工程研究, 2025, 29(7): 1475-1485. |
骨性关节炎通常根据Kellgren Lawrence (KL)分级采用阶梯方案治疗,常用的治疗手段包括药物治疗、膝关节部分置换术、人工膝关节置换术等。药物治疗只能暂时缓解骨性关节炎发病症状[6],关节置换术是临床针对中晚期骨性关节炎患者较为有效的治疗手段,但存在费用较高、假体寿命限制、手术风险等局限[7],研究对于骨性关节炎更为安全有效的治疗手段是一个值得深入研究的课题。近年来,干细胞疗法已成为骨性关节炎临床前和临床姑息治疗的一种选择,干细胞来源外泌体作为一种无细胞疗法有希望成为骨性关节炎的新型治疗手段。
外泌体是由细胞分泌携带各种细胞成分的细胞外囊泡,内含DNA、RNA、脂质、代谢物以及胞质和细胞表面蛋白等,见图1。外泌体在血管生成、细胞凋亡、抗原呈递、细胞间信号传导和炎症等活动中发挥重要功能,其通过细胞间通讯对受体细胞进行调控,是细胞间重要的通讯载体[8]。外泌体被内吞后将其自身携带的蛋白质、脂质以及核酸等细胞成分特异性转移到受体细胞中,从而引发后者的表型变化[9]。研究表明,骨关节滑液中可检测到外泌体,在骨性关节炎软骨细胞和相关炎症细胞的生物过程中起着至关重要的作用[10-11]。骨性关节炎患者滑液中的外泌体通过募集免疫细胞来维持滑膜炎症,刺激细胞产生白细胞介素1β和白细胞介素16 [12],同时刺激M1巨噬细胞释放参与炎症过程和软骨变性的关键分子,对炎症的延续及骨性关节炎的发生发展起关键作用[13]。外泌体通过转移miRNA和长链非编码RNA(long non-coding RNA,lncRNA)来调节骨性关节炎细胞的生物学功能[14],促进骨性关节炎中软骨的异常钙化和破坏以及诱导骨性关节炎关节软骨细胞的改变等[15-16]。缓解疼痛也是骨性关节炎治疗的关键目标之一,外泌体通过过表达相关基因,阻断软骨细胞与神经细胞的信号传导,从而缓解骨性关节炎带来的疼痛[17]。文章综述了近年来针对骨性关节炎的外泌体研究,旨在全面了解外泌体在骨性关节炎诊断中的可行性及不同干细胞来源外泌体在骨性关节炎治疗中的功能和机制,对工程化外泌体通过携载特定RNA加强其治疗效果进行总结,并对未来骨性关节炎患者外泌体治疗的前景和挑战进行讨论。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者于2023年10月进行检索。
1.1.2 检索文献时限 2003年1月至2023年10月。
1.1.3 检索数据库 PubMed数据库及中国知网。
1.1.4 检索词 英文检索词:“exosomes,osteoarthritis,mesenchymal stem cells,stem cells”,中文检索词:“外泌体,骨性关节炎,间充质干细胞,干细胞”。
1.1.5 检索文献类型 研究原著、综述、病例报告、荟萃分析等。
1.1.6 手工检索 无。
1.1.7 检索策略 见图2。
1.1.8 检索文献量 中文文献199篇,英文文献766篇。
1.2 纳入与排除标准
1.2.1 纳入标准 ①与干细胞外泌体在骨性关节炎治疗方面相关的文献;②对此次研究主题具有重要意义但年限久远的文献;③对相似文献尽量选取时间临近者。
1.2.2 排除标准 ①重复性研究;②相同研究类型但无新进展的文献;③发表于2004年以前的文献。
1.3 数据提取 共检索到文献965篇,经资料收集者对文章有效性和适用性进行评估,通过阅读题目与摘要进行初筛;排除与研究目的相关性差及内容陈旧、重复的文献,最终纳入99篇符合标准的文献进行综述,见图3。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
3.2 作者综述区别于他人他篇的特点 相较于其他相关综述,文章更加全面讨论了外泌体在骨性关节炎诊疗中的研究进展。在诊断方面,生物体液中的外泌体表现出作为新型生物标志物的巨大潜力。外泌体中所含的miRNA、lncRNA及蛋白和脂类都可作为生物标志物,诊断骨性关节炎发生的同时还可以评估骨性关节炎发展阶段,是骨性关节炎早期诊断的良好工具。在治疗方面,文章全面细致探讨了不同来源干细胞外泌体治疗骨性关节炎的作用机制及治疗进展。间充质干细胞来源外泌体由于本身具有的促进软骨细胞增殖及细胞外基质合成、缓解炎症及疼痛的能力,对骨性关节炎具有良好的治疗效果,通过过表达特定基因片段对其进行修饰,能够提高对骨性关节炎治疗的效果;胚胎干细胞和诱导多能干细胞由于无限增殖的特性,可能是未来量产外泌体更优异的来源;其他干细胞来源外泌体如hUSCs-Exos和ASCs-Exos也表现出治疗骨性关节炎的潜力。文章还结合了对外泌体修饰的创新研究,生物技术修饰具有增强其治疗效果的潜力。
3.3 综述的局限性 该综述的文献采用主题词结合自由词为主要检索方法,以确保尽可能广泛地覆盖相关文献。这种检索方法的选择,虽然能够涵盖多个方面和较长的时间跨度,但也可能导致文献检索不够充分。此外,由于筛选标准的主观性和多样性,可能存在一定的误差与偏倚。为了尽可能减少这些潜在问题,对检索到的文献进行了严格的筛选和评估。在综合考虑文献质量、研究方法和结果可靠性的基础上,归纳总结了最新研究内容和成果。尽管无法完全避免误差和偏倚,希望通过这种方式为读者提供一个全面而准确的研究概况。干细胞外泌体在骨性关节炎治疗领域的研究仍处于不断探索和发展的阶段。因此,该综述不仅是对现有研究成果的总结,更是对未来研究方向的一种展望。希望通过这篇综述,为干细胞外泌体治疗骨性关节炎的进一步研究提供思路。
3.4 综述的重要意义 研究不同来源干细胞的外泌体,对于更加深入了解骨性关节炎的发病机制至关重要。骨性关节炎作为一种复杂的关节疾病,其发病过程涉及多个生物过程和分子机制。而外泌体作为细胞间通讯的重要媒介,携带了丰富的生物信息,包括蛋白质、RNA和其他生物活性分子。通过深入了解骨性关节炎发病过程中细胞间的相互作用和信号传递,从而找到潜在的治疗靶点。
未来有望利用外泌体内部RNA、蛋白质及脂类的动态变化特征,精准预测骨性关节炎的进程。通过实时监测这些成分的细微变动,及时了解病情的发展变化,从而更加客观地评估骨性关节炎的治疗效果,并根据实际情况灵活调整和优化治疗方案,为临床医生提供更加精准、个性化的医疗手段。
工程化外泌体的开发旨在突破传统外泌体在医学应用中的局限,以推进其在疾病治疗中的应用。通过负载特定RNA,工程化外泌体增强了治疗的特异性和效率并提升其荷载能力,使其能高效携带并递送治疗分子。工程化方法给予未来外泌体的标准化和规模化生产的可能,在医学领域具有广阔的应用前景。现有研究工程化外泌体的RNA编辑多聚焦于miRNA,miRNA是一类非编码RNA分子,它们通过影响mRNA的稳定性和翻译来调节基因表达。一个特定的miRNA可以针对多个基因产生调节作用,从而在骨性关节炎的进展中发挥重要作用,外泌体在疾病中的作用既可以依赖于单个的miRNA,也可以依赖于其内含的多个miRNA一起发挥作用。工程化外泌体通过不同的信号途径和靶点产生协同效应,对骨性关节炎的治疗起到积极作用。通过确定外泌体中具体的RNA及其作用机制,研究人员能够解释细胞间通信的精确分子基础,从而深入理解疾病发生、发展及治疗,为骨性关节炎的早期诊断、预后评估及靶向治疗提供了重要依据。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
外泌体:是由细胞分泌携带各种细胞成分的细胞外囊泡,包括DNA、RNA、脂质、代谢物以及胞质和细胞表面蛋白,与免疫反应、病毒致病性、妊娠、心血管疾病、中枢神经系统相关疾病和癌症等多种生理和病理过程相关。#br#非编码RNA:指由基因组转录而成的不编码蛋白质的 RNA 分子。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
外泌体相关研究近年来备受瞩目,其在骨性关节炎的诊疗领域展现出巨大的潜力。利用外泌体内部成分动态变化,精准监测病情进展,客观评估治疗效果,为临床提供精准、个性化治疗手段。工程化外泌体则突破传统局限,通过负载特定RNA增强治疗特异性与效率,展现出广阔的前景。明确外泌体中RNA作用机制,有助于早期诊断、预后评估及靶向治疗,为骨性关节炎的精准医疗奠定重要基础。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||