[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
[2] ZHANG A, MIAO K, SUN H, et al. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int J Biol Sci. 2022;18(7):3019-3033.
[3] HAN QF, LI WJ, HU KS, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022; 21(1):207.
[4] HOSSEINIKHAH SM, GHEYBI F, MOOSAVIAN SA, et al. Role of exosomes in tumour growth, chemoresistance and immunity: state-of-the-art. J Drug Target. 2023;31(1):32-50.
[5] HILL M, TRAN N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14(4):dmm047662.
[6] JAFARI A, BABAJANI A, ABDOLLAHPOUR-ALITAPPEH M, et al. Exosomes and cancer: from molecular mechanisms to clinical applications. Med Oncol. 2021;38(4):45.
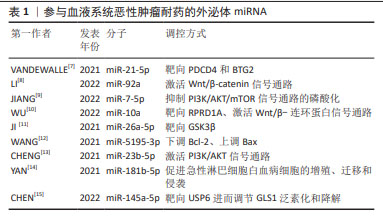
[7] VANDEWALLE V, ESSAGHIR A, BOLLAERT E, et al. miR-15a-5p and miR-21-5p contribute to chemoresistance in cytogenetically normal acute myeloid leukaemia by targeting PDCD4, ARL2 and BTG2. J Cell Mol Med. 2021;25(1):575-585.
[8] LI H, XIE C, LU Y, et al. Exosomal miR92a Promotes Cytarabine Resistance in Myelodysplastic Syndromes by Activating Wnt/β-catenin Signal Pathway. Biomolecules. 2022;12(10):1448.
[9] JIANG D, WU X, SUN X, et al. Bone mesenchymal stem cell-derived exosomal microRNA-7-5p inhibits progression of acute myeloid leukemia by targeting OSBPL11. J Nanobiotechnology. 2022;20(1):29.
[10] WU J, ZHANG Y, LI X, et al. Exosomes from bone marrow mesenchymal stem cells decrease chemosensitivity of acute myeloid leukemia cells via delivering miR-10a. Biochem Biophys Res Commun. 2022;622: 149-156.
[11] JI D, HE Y, LU W, et al. Small-sized extracellular vesicles (EVs) derived from acute myeloid leukemia bone marrow mesenchymal stem cells transfer miR-26a-5p to promote acute myeloid leukemia cell proliferation, migration, and invasion. Hum Cell. 2021;34(3):965-976.
[12] WANG D, MING X, XU J, et al. Circ_0009910 shuttled by exosomes regulates proliferation, cell cycle and apoptosis of acute myeloid leukemia cells by regulating miR-5195-3p/GRB10 axis. Hematol Oncol. 2021;39(3):390-400.
[13] CHENG H, DING J, TANG G, et al. Human mesenchymal stem cells derived exosomes inhibit the growth of acute myeloid leukemia cells via regulating miR-23b-5p/TRIM14 pathway. Mol Med. 2021; 27(1):128.
[14] YAN W, SONG L, WANG H, et al. Extracellular vesicles carrying miRNA-181b-5p affects the malignant progression of acute lymphoblastic leukemia. J Transl Med. 2021;19(1):511.
[15] CHEN X, CHEN Y, ZHANG M, et al. HucMSC exosomes promoted imatinib-induced apoptosis in K562-R cells via a miR-145a-5p/USP6/GLS1 axis. Cell Death Dis. 2022;13(1):92.
[16] CAO D, CAO X, JIANG Y, et al. Circulating exosomal microRNAs as diagnostic and prognostic biomarkers in patients with diffuse large B-cell lymphoma. Hematol Oncol. 2022;40(2):172-180.
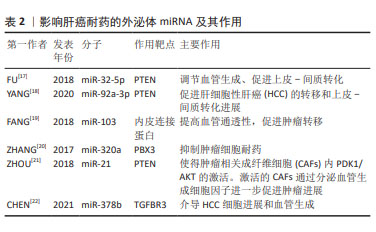
[17] FU X, LIU M, QU S, et al. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37(1):52.
[18] YANG B, FENG X, LIU H, et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene. 2020;39(42): 6529-6543.
[19] FANG JH, ZHANG ZJ, SHANG LR, et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68(4): 1459-1475.
[20] ZHANG Z, LI X, SUN W, et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33-42.
[21] ZHOU Y, REN H, DAI B, et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37(1):324.
[22] CHEN W, HUANG L, LIANG J, et al. Hepatocellular carcinoma cells-derived exosomal microRNA-378b enhances hepatocellular carcinoma angiogenesis. Life Sci. 2021;273:119184.
[23] MARTÍN-SÁNCHEZ JC, LUNET N, GONZÁLEZ-MARRÓN A, et al. Projections in Breast and Lung Cancer Mortality among Women: A Bayesian Analysis of 52 Countries Worldwide. Cancer Res. 2018; 78(15):4436-4442.
[24] WEI F, MA C, ZHOU T, et al. Correction to: Exosomes derived from gemcitabine resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2021;20(1):35.
[25] SUN S, CHEN H, XU C, et al. Exosomal miR-106b serves as a novel marker for lung cancer and promotes cancer metastasis via targeting PTEN. Life Sci. 2020;244:117297.
[26] DONG C, LIU X, WANG H, et al. Hypoxic non-small-cell lung cancer cell-derived exosomal miR-21 promotes resistance of normoxic cell to cisplatin. Onco Targets Ther. 2019;12:1947-1956.
[27] SONG Z, JIA G, MA P, et al. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021;276:119399.
[28] MAO G, LIU Y, FANG X, et al. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis. 2015;18(3): 373-382.
[29] MA Y, YUWEN D, CHEN J, et al. Exosomal Transfer Of Cisplatin-Induced miR-425-3p Confers Cisplatin Resistance In NSCLC Through Activating Autophagy. Int J Nanomedicine. 2019;14:8121-8132.
[30] SCHRAMM F, SCHAEFER L, WYGRECKA M. EGFR Signaling in Lung Fibrosis. Cells. 2022;11(6):986.
[31] ZHANG Y, LI M, HU C. Exosomal transfer of miR-214 mediates gefitinib resistance in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;507(1-4):457-464.
[32] LEONETTI A, CAPULA M, MINARI R, et al. Dynamic Evaluation of Circulating miRNA Profile in EGFR-Mutated NSCLC Patients Treated with EGFR-TKIs. Cells. 2021;10(6):1520.
[33] XAVIER CPR, BELISARIO DC, REBELO R, et al. The role of extracellular vesicles in the transfer of drug resistance competences to cancer cells. Drug Resist Updat. 2022;62:100833.
[34] LEHUÉDÉ C, LI X, DAUVILLIER S, et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP). Breast Cancer Res. 2019;21(1):7.
[35] BIGAGLI E, CINCI L, D’AMBROSIO M, et al. Transcriptomic Characterization, Chemosensitivity and Regulatory Effects of Exosomes in Spontaneous EMT/MET Transitions of Breast Cancer Cells. Cancer Genomics Proteomics. 2019;16(3):163-173.
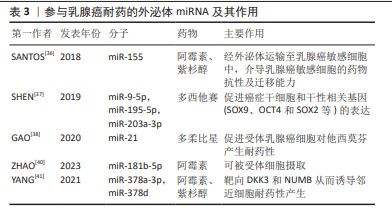
[36] SANTOS JC, LIMA NDS, SARIAN LO, et al. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep. 2018;8(1):829.
[37] SHEN M, DONG C, RUAN X, et al. Chemotherapy-Induced Extracellular Vesicle miRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer Res. 2019;79(14):3608-3621.
[38] GAO Y, LI X, ZENG C, et al. CD63+ Cancer-Associated Fibroblasts Confer Tamoxifen Resistance to Breast Cancer Cells through Exosomal miR-22. Adv Sci (Weinh). 2020;7(21):2002518.
[39] NING K, WANG T, SUN X, et al. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J Surg Oncol. 2017;115(8):932-940.
[40] ZHAO S, PAN T, DENG J, et al. Exosomal transfer of miR-181b-5p confers senescence-mediated doxorubicin resistance via modulating BCLAF1 in breast cancer. Br J Cancer. 2023;128(4):665-677.
[41] YANG Q, ZHAO S, SHI Z, et al. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling. J Exp Clin Cancer Res. 2021;40(1):120.
[42] BOUSSIOS S, RASSY E, SAMARTZIS E, et al. Melanoma of unknown primary: New perspectives for an old story. Crit Rev Oncol Hematol. 2021;158:103208.
[43] ZHANG Z, RICHMOND A, YAN C. Immunomodulatory Properties of PI3K/AKT/mTOR and MAPK/MEK/ERK Inhibition Augment Response to Immune Checkpoint Blockade in Melanoma and Triple-Negative Breast Cancer. Int J Mol Sci. 2022;23(13):7353.
[44] VELLA LJ, BEHREN A, COLEMAN B, et al. Intercellular Resistance to BRAF Inhibition Can Be Mediated by Extracellular Vesicle-Associated PDGFRβ. Neoplasia. 2017;19(11):932-940.
[45] LUNAVAT TR, CHENG L, EINARSDOTTIR BO, et al. BRAFV600 inhibition alters the microRNA cargo in the vesicular secretome of malignant melanoma cells. Proc Natl Acad Sci U S A. 2017;114(29):E5930-E5939.
[46] GEBHARDT K, EDEMIR B, GROß E, et al. BRAF/EZH2 Signaling Represses miR-129-5p Inhibition of SOX4 Thereby Modulating BRAFi Resistance in Melanoma. Cancers (Basel). 2021;13(10):2393.
[47] CHEN H, ZENG B, LI X, et al. High-Metastatic Melanoma Cells Promote the Metastatic Capability of Low-Metastatic Melanoma Cells via Exosomal Transfer of miR-411-5p. Front Oncol. 2022;12:895164.
[48] SHELTON M, ANENE CA, NSENGIMANA J, et al. The role of CAF derived exosomal microRNAs in the tumour microenvironment of melanoma. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188456.
[49] PALACIOS-FERRER JL, GARCÍA-ORTEGA MB, GALLARDO-GÓMEZ M, et al. Metabolomic profile of cancer stem cell-derived exosomes from patients with malignant melanoma. Mol Oncol. 2021;15(2):407-428.
[50] TRUFFI M, SORRENTINO L, CORSI F. Fibroblasts in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1234:15-29.
[51] SHU S, YANG Y, ALLEN CL, et al. Publisher Correction: Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep. 2019;9(1):4959.
[52] ARNOLD M, SIERRA MS, LAVERSANNE M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66(4):683-691.
[53] LA VECCHIA S, SEBASTIÁN C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin Cell Dev Biol. 2020;98:63-70.
[54] MALEKI M, GOLCHIN A, JAVADI S, et al. Role of exosomal miRNA in chemotherapy resistance of Colorectal cancer: A systematic review. Chem Biol Drug Des. 2023;101(5):1096-1112.
[55] ZHANG HW, SHI Y, LIU JB, et al. Cancer-associated fibroblast-derived exosomal microRNA-24-3p enhances colon cancer cell resistance to MTX by down-regulating CDX2/HEPH axis. J Cell Mol Med. 2021; 25(8):3699-3713.
[56] HU JL, WANG W, LAN XL, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18(1):91.
[57] SANTOS P, ALMEIDA F. Role of Exosomal miRNAs and the Tumor Microenvironment in Drug Resistance. Cells. 2020;9(6):1450.
[58] GAO R, FANG C, XU J, et al. LncRNA CACS15 contributes to oxaliplatin resistance in colorectal cancer by positively regulating ABCC1 through sponging miR-145. Arch Biochem Biophys. 2019; 663:183-191.
[59] JIN G, LIU Y, ZHANG J, et al. A panel of serum exosomal microRNAs as predictive markers for chemoresistance in advanced colorectal cancer. Cancer Chemother Pharmacol. 2019;84(2):315-325.
[60] YAO S, YIN Y, JIN G, et al. Exosome-mediated delivery of miR-204-5p inhibits tumor growth and chemoresistance. Cancer Med. 2020; 9(16):5989-5998. |