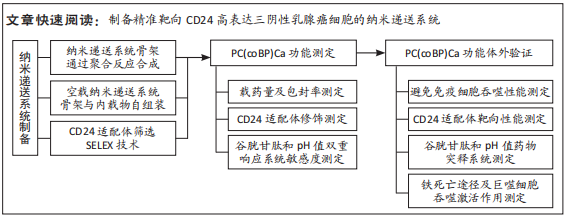
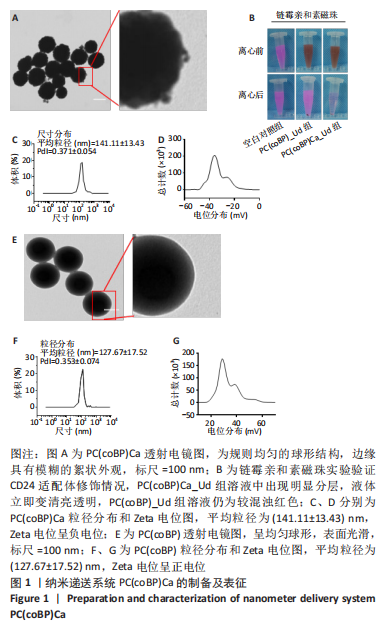
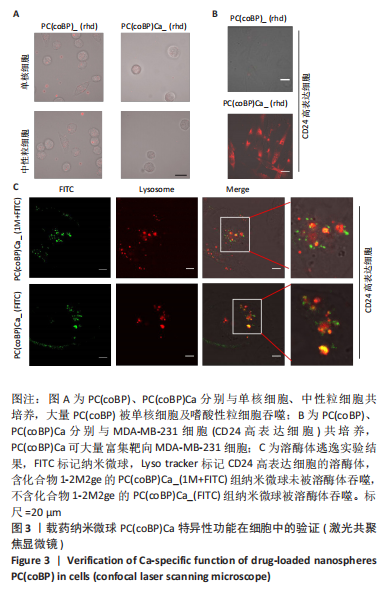
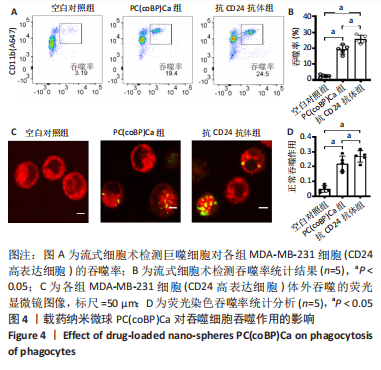
[1] SCHNEIDER BP, JIANG G, BALLINGER TJ, et al. BRE12-158: A Postneoadjuvant, Randomized Phase II Trial of Personalized Therapy Versus Treatment of Physician’s Choice for Patients With Residual Triple-Negative Breast Cancer. J Clin Oncol. 2022;40(4):345-355.
[2] BIANCHINI G, DE ANGELIS C, LICATA L, et al. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19(2):91-113.
[3] HEISER LM, MILLS GB, GRAY JW. Therapeutic Clues from an Integrated Omic Assessment of East Asian Triple Negative Breast Cancers. Cancer Cell. 2019;35(3):341-343.
[4] CONFORTI F, PALA L, SALA I, et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ. 2021;375:e066381.
[5] GUO Z, HE H, ZHANG Y, et al. Heavy-Atom-Modulated Supramolecular Assembly Increases Antitumor Potency against Malignant Breast Tumors via Tunable Cooperativity. Adv Mater. 2021;33(2):e2004225.
[6] MU QG, LIN G, JEON M, et al. Iron oxide nanoparticle targeted chemo-immunotherapy for triple negative breast cancer. Mater Today (Kidlington. 2021;50:149-169.
[7] SCHMID P, ADAMS S, RUGO HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379(20): 2108-2121.
[8] DAVIS ME, CHEN ZG, SHIN DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771-782.
[9] WANG T, ZHANG J, HOU T, et al. Selective targeting of tumor cells and tumor associated macrophages separately by twin-like core-shell nanoparticles for enhanced tumor-localized chemoimmunotherapy. Nanoscale. 2019;11(29):13934-13946.
[10] YANG G, LIU Y, WANG H, et al. Bioinspired Core-Shell Nanoparticles for Hydrophobic Drug Delivery. Angew Chem Int Ed Engl. 2019;58(40): 14357-14364.
[11] LIANG S, HU J, XIE Y, t al. A polyethylenimine-modified carboxyl-poly(styrene/acrylamide) copolymer nanosphere for co-delivering of CpG and TGF-β receptor I inhibitor with remarkable additive tumor regression effect against liver cancer in mice. Int J Nanomedicine. 2016;11:6753-6762.
[12] MI X, HU M, DONG M, et al. Folic Acid Decorated Zeolitic Imidazolate Framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int J Nanomedicine. 2021;16:8337-8352.
[13] ZHAO Q, SUN X, WU B, et al. Construction of homologous cancer cell membrane camouflage in a nano-drug delivery system for the treatment of lymphoma. J Nanobiotechnol. 2021;19(1):8.
[14] DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072.
[15] YU H, GUO P, XIE X, et al. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21(4): 648-657.
[16] TOYOKUNI S. Iron addiction with ferroptosis-resistance in asbestos-induced mesothelial carcinogenesis: Toward the era of mesothelioma prevention. Free Radic Biol Med. 2019;133:206-215.
[17] ZUO T, FANG T, ZHANG J, et al. pH-Sensitive Molecular-Switch-Containing Polymer Nanoparticle for Breast Cancer Therapy with Ferritinophagy-Cascade Ferroptosis and Tumor Immune Activation. Adv Healthc Mater. 2021;10(21):e2100683.
[18] LI J, LI J, PU Y, et al. PDT-Enhanced Ferroptosis by a Polymer Nanoparticle with pH-Activated Singlet Oxygen Generation and Superb Biocompatibility for Cancer Therapy. Biomacromolecules. 2021;22(3): 1167-1176.
[19] TOYOKUNI S, YANATORI I, KONG Y, et al. Ferroptosis at the crossroads of infection, aging and cancer. Cancer Sci. 2020;111:2665-2671.
[20] YAGODA N, VON RECHENBERG M, ZAGANJOR E, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864-868.
[21] ZHENG H, JIANG J, XU S, et al. Nanoparticle-induced ferroptosis: detection methods, mechanisms and applications. Nanoscale. 2021; 13(4):2266-2285.
[22] LI Y, WANG X, YAN J, et al. Nanoparticle ferritin-bound erastin and rapamycin: a nanodrug combining autophagy and ferroptosis for anticancer therapy. Biomater Sci. 2019;7(9):3779-3787.
[23] HOU L, PU L, CHEN Y, et al. Targeted Intervention of NF2–YAP Signaling Axis in CD24-Overexpressing Cells Contributes to Encouraging Therapeutic Effects in TNBC. ACS Nano. 2022. doi: 10.1021/acsnano. 1c10921.
[24] BIANCHINI G, BALKO JM, MAYER IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(10):674-690.
[25] LIANG C, ZHANG X, YANG M, et al. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater. 2019;31(51):e1904197.
[26] SHEN Z, SONG J, YUNG BC, et al. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv Mater. 2018;30(12):e1704007.
[27] LIU Y, WANG J, XIONG Q, et al. Nano-Bio Interactions in Cancer: From Therapeutics Delivery to Early Detection. Acc Chem Res. 2021;54(2): 291-301.
[28] LI S, BENNETT ZT, SUMER BD, et al. Nano-Immune-Engineering Approaches to Advance Cancer Immunotherapy: Lessons from Ultra-pH-Sensitive Nanoparticles. Acc Chem Res. 2020;53:2546-2557.
[29] LANG T, LIU Y, ZHENG Z, et al. Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv Mater. 2019;31(33):e1806202.
[30] 朱仔巍,代英辉,王东凯.金属有机框架及其载运抗肿瘤药物的研究进展[J].中国药剂学杂志(网络版),2022,20(2):75-82.
[31] RAZZAQUE S, GUO L, WENG J, et al. Facile fabrication of hypercrosslinked microporous polymer nanospheres for effective inhibition of triple negative breast cancer cells proliferation. J Colloid Interface Sci. 2022;620:94-106.
[32] XU S, YANG Q, WANG R, et al. Genetically engineered pH-responsive silk sericin nanospheres with efficient therapeutic effect on ulcerative colitis. Acta Biomater. 2022;144:81-95.
[33] YANG D, LIU M, XU J, et al. Carbon nanosphere-based fluorescence aptasensor for targeted detection of breast cancer cell MCF-7. Talanta. 2018;185:113-117.
[34] LIANG P, ZHENG J, DAI S, et al. pH triggered re-assembly of nanosphere to nanofiber: The role of peptide conformational change for enhanced cancer therapy. J Control Release. 2017;260:22-31.
[35] YANG HY, LI Y, LEE DS. Recent Advances of pH-Induced Charge-Convertible Polymer-Mediated Inorganic Nanoparticles for Biomedical Applications. Macromol Rapid Commun. 2020;41(21):e2000106.
[36] LI S, ZHANG Y, HO SH, et al. Combination of tumour-infarction therapy and chemotherapy via the co-delivery of doxorubicin and thrombin encapsulated in tumour-targeted nanoparticles. Nat Biomed Eng. 2020;4(7): 732-742.
[37] LI Z, DI C, LI S, et al. Smart Nanotherapeutic Targeting of Tumor Vasculature. Acc Chem Res. 2019;52(9):2703-2712.
[38] ANGELI J PF, SCHNEIDER M, PRONETH B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180-1191.
[39] WU J, MINIKES AM, GAO M, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769): 402-406.
[40] TU TT, SUN Y, LEI YM, et al. Pyrenecarboxaldehyde encapsulated porous TiO nanoreactors for monitoring cellular GSH levels. Nanoscale. 2022;14(15):5751-5757. |