中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (35): 5664-5669.doi: 10.3969/j.issn.2095-4344.1955
• 组织构建临床实践 clinical practice in tissue construction • 上一篇 下一篇
重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白递减联合沙利度胺递增治疗方案干预活动性强直性脊柱炎的1年评价
周 进,付 林,周 珍,李秋蓉
- (四川省宜宾市第二人民医院风湿免疫科,四川省宜宾市 644000)
One-year evaluation of recombinant human tumor necrosis factor-alpha receptor II decrement combined with thalidomide increment in the treatment of active ankylosing spondylitis
Zhou Jin, Fu Lin, Zhou Zhen, Li Qiurong
- (Department of Rheumatism and Immunology, the Second People’s Hospital of Yibin, Yibin 644000, Sichuan Province, China)
摘要:
文章快速阅读:
.jpg) 文题释义:
Bath强直性脊柱炎疾病活动性指数(BASDAI):评价内容包括6项:即患者过去1周的身体疲倦程度、颈背部髋关节的疼痛程度、其他关节的疼痛或肿胀程度、肌腱端炎、晨僵程度及时间。评定方法:每项得分为患者自我评价的VAS(0-10)评分,BASDAI=0.2×[第1项+第2项+第3项+第4项+0.5×(第5项+第6项)]。
ASDAS-CRP:基于C-反应蛋白强直性脊柱炎疾病活动度评分(ASDAS-CRP)=0.121×腰背痛+0.058×晨僵持续时间+0.110×患者总体评价+ 0.073×外周关节疼痛/肿胀+0.579×Ln(CRP+1);ASDAS-CRP 计算后得到的精度比较高,且与患者病情活动度之间存在的相关性要明显优于BASDAI,显示出ADSAS 相比 BASDAI 更能够反映患者的活动度情况。
文题释义:
Bath强直性脊柱炎疾病活动性指数(BASDAI):评价内容包括6项:即患者过去1周的身体疲倦程度、颈背部髋关节的疼痛程度、其他关节的疼痛或肿胀程度、肌腱端炎、晨僵程度及时间。评定方法:每项得分为患者自我评价的VAS(0-10)评分,BASDAI=0.2×[第1项+第2项+第3项+第4项+0.5×(第5项+第6项)]。
ASDAS-CRP:基于C-反应蛋白强直性脊柱炎疾病活动度评分(ASDAS-CRP)=0.121×腰背痛+0.058×晨僵持续时间+0.110×患者总体评价+ 0.073×外周关节疼痛/肿胀+0.579×Ln(CRP+1);ASDAS-CRP 计算后得到的精度比较高,且与患者病情活动度之间存在的相关性要明显优于BASDAI,显示出ADSAS 相比 BASDAI 更能够反映患者的活动度情况。
.jpg) 文题释义:
Bath强直性脊柱炎疾病活动性指数(BASDAI):评价内容包括6项:即患者过去1周的身体疲倦程度、颈背部髋关节的疼痛程度、其他关节的疼痛或肿胀程度、肌腱端炎、晨僵程度及时间。评定方法:每项得分为患者自我评价的VAS(0-10)评分,BASDAI=0.2×[第1项+第2项+第3项+第4项+0.5×(第5项+第6项)]。
ASDAS-CRP:基于C-反应蛋白强直性脊柱炎疾病活动度评分(ASDAS-CRP)=0.121×腰背痛+0.058×晨僵持续时间+0.110×患者总体评价+ 0.073×外周关节疼痛/肿胀+0.579×Ln(CRP+1);ASDAS-CRP 计算后得到的精度比较高,且与患者病情活动度之间存在的相关性要明显优于BASDAI,显示出ADSAS 相比 BASDAI 更能够反映患者的活动度情况。
文题释义:
Bath强直性脊柱炎疾病活动性指数(BASDAI):评价内容包括6项:即患者过去1周的身体疲倦程度、颈背部髋关节的疼痛程度、其他关节的疼痛或肿胀程度、肌腱端炎、晨僵程度及时间。评定方法:每项得分为患者自我评价的VAS(0-10)评分,BASDAI=0.2×[第1项+第2项+第3项+第4项+0.5×(第5项+第6项)]。
ASDAS-CRP:基于C-反应蛋白强直性脊柱炎疾病活动度评分(ASDAS-CRP)=0.121×腰背痛+0.058×晨僵持续时间+0.110×患者总体评价+ 0.073×外周关节疼痛/肿胀+0.579×Ln(CRP+1);ASDAS-CRP 计算后得到的精度比较高,且与患者病情活动度之间存在的相关性要明显优于BASDAI,显示出ADSAS 相比 BASDAI 更能够反映患者的活动度情况。摘要
背景:肿瘤坏死因子拮抗剂(如益赛普、阿达木单抗等)是目前治疗活动期强直性脊柱炎的最佳选择,但此类药物价格昂贵,寻求有效价廉的替代方案势在必行。
目的:探讨重组人Ⅱ型肿瘤坏死因子受体-抗体融合蛋白(益赛普)递减应用联合沙利度胺递增治疗活动性强直性脊柱炎的1年随访。
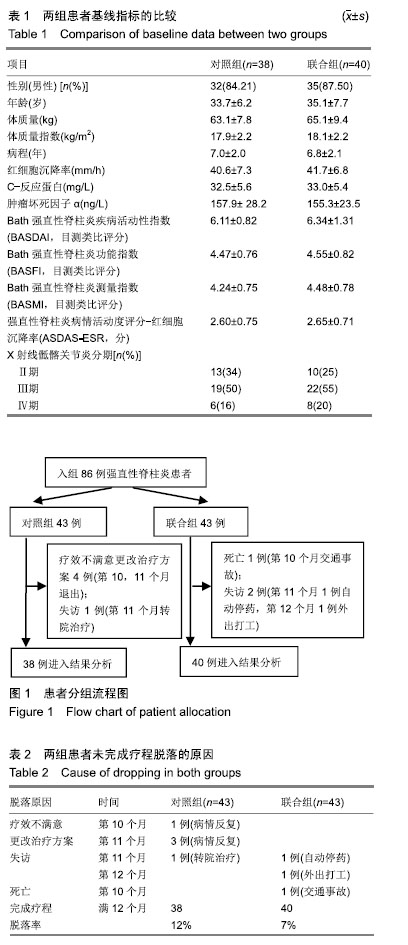
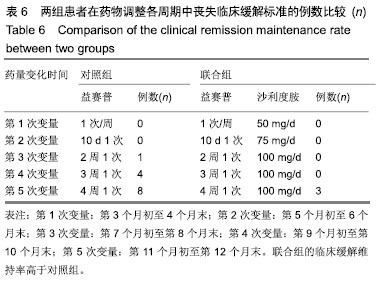
方法:研究经宜宾市第二人民医院医学伦理委员会审核批准。86例活动期强直性脊柱炎患者随机分为对照组和联合组各43例,患者及家属对治疗方案均知情同意。2组患者均用益赛普皮下注射,均25 mg/次:初始用药,2次/周,连用2个月;当病情达到临床缓解标准后,即将益赛普每隔2个月递减剂量:第3个月初由2次/周减至1次/周;第5个月初减至10 d 1次;第7个月初减至2周1次;第9个月初减至3周1次;第11个月初减至4周1次,此后不再减量,用至12月末。若每次减量使病情加重而达不到临床缓解标准,则将益赛普重新调回前一个剂量。联合组患者同时联用沙利度胺片睡前服用,1次/d。初始剂量25 mg/d,连用2个月,以后每隔2个月剂量递增25 mg/d,直到第7个月初达到100 mg/d时不再增加。2组患者疗程为12个月。
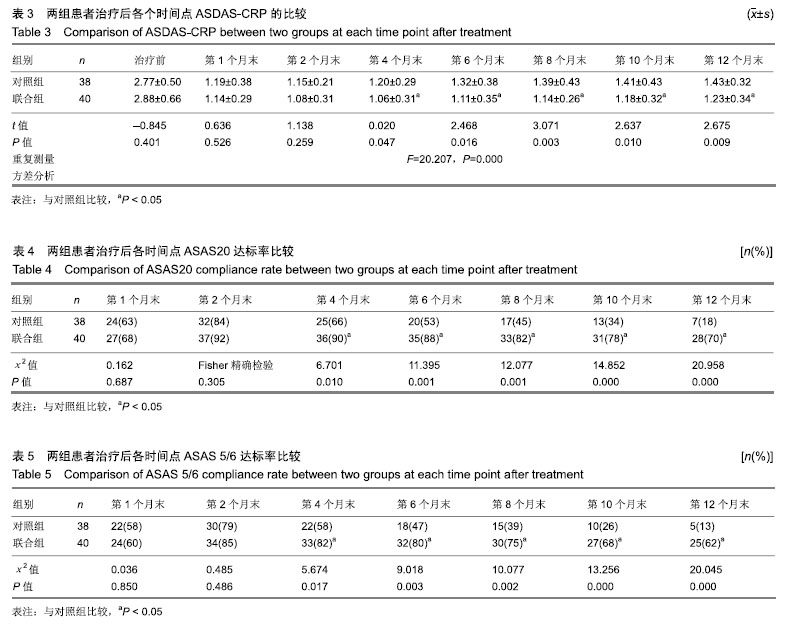
结果与结论:①对照组43例,完成12个月疗程38例;联合组43例,完成12个月疗程40例;②从治疗后第4个月末至第12个月末,联合组的基于C-反应蛋白强直性脊柱炎疾病活动度评分(ASDAS-CRP)评分均显著低于对照组(P < 0.05或P < 0.01);③联合组的20%改善程度(ASAS20)达标率和强直性脊柱炎疗效评价ASAS 5/6达标率均高于对照组(P < 0.05或P < 0.01);④联合组的临床缓解维持率高于对照组(χ2=8.527,P=0.003);⑤12个月内联合组每位患者益赛普总用药量低于对照组(t=2.932,P=0.004);⑥2组患者不良反应发生率比较差异无显著性意义(χ2=0.174,P=0.677);⑦结果说明,益赛普递减联合沙利度胺递增治疗强直性脊柱炎12个月,在延长益赛普用药间隔的方案中,可取得较高的疾病缓解率或低疾病活动状态,沙利度胺联合益赛普并不增加药物的不良反应。
中图分类号:
.jpg) 文题释义:
Bath强直性脊柱炎疾病活动性指数(BASDAI):评价内容包括6项:即患者过去1周的身体疲倦程度、颈背部髋关节的疼痛程度、其他关节的疼痛或肿胀程度、肌腱端炎、晨僵程度及时间。评定方法:每项得分为患者自我评价的VAS(0-10)评分,BASDAI=0.2×[第1项+第2项+第3项+第4项+0.5×(第5项+第6项)]。
ASDAS-CRP:基于C-反应蛋白强直性脊柱炎疾病活动度评分(ASDAS-CRP)=0.121×腰背痛+0.058×晨僵持续时间+0.110×患者总体评价+ 0.073×外周关节疼痛/肿胀+0.579×Ln(CRP+1);ASDAS-CRP 计算后得到的精度比较高,且与患者病情活动度之间存在的相关性要明显优于BASDAI,显示出ADSAS 相比 BASDAI 更能够反映患者的活动度情况。
文题释义:
Bath强直性脊柱炎疾病活动性指数(BASDAI):评价内容包括6项:即患者过去1周的身体疲倦程度、颈背部髋关节的疼痛程度、其他关节的疼痛或肿胀程度、肌腱端炎、晨僵程度及时间。评定方法:每项得分为患者自我评价的VAS(0-10)评分,BASDAI=0.2×[第1项+第2项+第3项+第4项+0.5×(第5项+第6项)]。
ASDAS-CRP:基于C-反应蛋白强直性脊柱炎疾病活动度评分(ASDAS-CRP)=0.121×腰背痛+0.058×晨僵持续时间+0.110×患者总体评价+ 0.073×外周关节疼痛/肿胀+0.579×Ln(CRP+1);ASDAS-CRP 计算后得到的精度比较高,且与患者病情活动度之间存在的相关性要明显优于BASDAI,显示出ADSAS 相比 BASDAI 更能够反映患者的活动度情况。